Beacon Tip Angiographic Catheter
Beacon Tip Angiographic Catheters are long thin plastic tubes inserted into an artery through a small incision in the skin. Health care providers use them in the peripheral and coronary vascular system to inject a contrast dye into the bloodstream for angiographic procedures. In 2015 and 2016, Cook Medical issued a recall because of reports that the tips were breaking.
Cook Medical received U.S. Food and Drug Administration clearance for one of its first Beacon Tip catheter models, the Slip-Cath Beacon Tip Catheter, in 2012.
Doctors use flexible catheters to inject dye into blood vessels during diagnostic angiograms. Beacon Tip catheters have distal tips that are designed to help doctors see better and maneuver the catheter better inside the body.
In 2015, the manufacturer of Beacon Tip Angiographic Catheters voluntarily recalled specific lots of the device after reports had surfaced of catheter tips splitting or separating. Cook Medical expanded the recall to all catheters with the Beacon Tip technology in 2016. The Class 1 recall, the most serious kind, involved more than 4 million catheters.
This problem created the possibility of catheter tips entering patients’ blood vessels, causing serious injury or death and requiring medical intervention to retrieve the tip. Additionally, there was the probability of the device malfunctioning due to the splitting or separation.
In April 2018, Cook announced the relaunch of the Beacon® Tip Torcon NB Advantage 5 Fr Catheter in the United States.
What Is a Catheter Angiography?
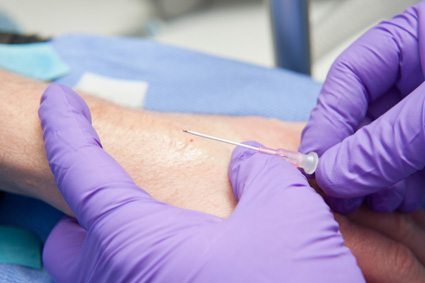
A catheter angiography requires the use of a catheter, X-ray imaging and often an injection of contrast dye to examine blood vessels in the body for abnormalities, such as plaque and other blockages, aneurysms, spasms or narrowing. The procedure is usually minimally invasive as well as efficient by assisting in the simultaneous diagnosis and treatment of a patient, thereby limiting patient exposure and back-to-back procedures.
A catheter angiography can be used to examine blood vessels throughout various components of the body including the respiratory system, digestive system, kidneys and urinary system, cardiovascular system, brain, neck, pelvis, legs and feet, arms and hands. The area where the catheter is to be inserted is shaved, cleaned and often numbed with a local anesthetic unless sedation is required. Catheter angiographies can last anywhere from less than an hour to several hours depending on the patient and the nature of the procedure.
Intended Angiographic Catheter Uses
Beacon Tip Angiographic Catheters were intended for use in the peripheral and coronary vascular system, including the carotid arteries, by physicians and qualified health care professionals in angiographic procedures.
- Beacon Tip Torcon NB Advantage Catheter
- By physicians and qualified health care professionals in the peripheral and coronary vascular system, including the carotid arteries, in angiographic procedures
- Beacon Tip Royal Flush Plus High-Flow Catheter
- By physicians and qualified health care professionals in angiographic procedures
- BeaconTip Centimeter Sizing Catheter
- By physicians and qualified health care professionals in angiographic procedures
- Beacon Tip White Vessel Sizing Catheter
- By physicians and qualified health care professionals in angiographic procedures
- Beacon Tip Vessel Sizing Catheter
- By physicians and qualified health care professionals in angiographic procedures
- Shuttle Select Slip-Cath
- By physicians and qualified health care professionals in angiographic procedures
- Slip-Cath Beacon® Tip Catheter
- By physicians and qualified health care professionals in angiographic procedures
- FluoroSet Radiographic Tubal Assessment Set
- Instillation of contrast dye into the uterine cavity for radiographic evaluation; injection of appropriate contrast dye into the fallopian tubes for evaluation of tubal patency
- Haskal Transjugular Intrahepatic Portal Access Set
- Transjugular liver access in diagnostic and interventional procedures
- Kumpe Access Catheter
- Used in combination with a HiWire, Bentson or other flexible-tipped wire guide to gain difficult ureteral access
- Liver Access and Biopsy Needle Set
- Obtaining liver histology samples via a jugular vein approach
- Neff D’Agostino Percutaneous Access Set
- Single-puncture percutaneous access to facilitate placement of a .038 inch (0.97 mm) diameter working wire guide for interventional radiology procedures
- Aprima Access Nonvascular Introducer Set
- Single-puncture percutaneous access to facilitate placement of a .038 inch (0.97 mm) diameter working wire guide for interventional radiology procedures
- Selective Salpingography Catheter with Beacon Tip
- Injection of contrast medium into the fallopian tube(s) for selective salpingography
- Transluminal Biliary Biopsy Forceps Set
- Access to and biopsy of tissue within the biliary ductal system
- White Lumax Guiding Coaxial Catheter
- Delivery of angioplasty balloons and other types of interventional devices
Side Effects and Complications
Cook Medical received frequent reports of a problem called “polymer degradation.” That caused catheter tips to break. The catheters underwent a preliminary investigation that identified environmental conditions, such as storage temperature, humidity and the use of Vaporized Hydrogen Peroxide (VHP) for whole-room decontamination within health care facilities, as possible contributing factors to the malfunction.
- Loss of device function
- Separation of a device segment leading to medical intervention
- Complications resulting from a separated segment, such as device fragments lodging in the vascular system
- Heart embolism (blockage of blood vessel in the heart)
- Lung embolism (blockage of blood vessel in the lung)
- Occluding (restricting/blocking) blood flow to end organs
Polymer Degradation and Voluntary Recall Timeline
Cook Medical decided to voluntarily recall its product following what it described as “an increase in reports of polymer degradation of the catheter tip, resulting in tip fracture and/or separation.”
Polymer degradation is the breakdown of material from heat, oxygen and mechanical stress. Other environmental causes can cause the failure.
The company initially received 26 reports of the device malfunctioning, with 14 of those reports resulting in adverse events. That prompted a lot-specific recall on July 2, 2015. The recall was expanded in October 2015 for all lots of select sizes of catheters. It was expanded again in April 2016 to include all lots of catheters with Beacon Tip technology.
The voluntary global recall ultimately included a total of 4,146,309 catheters. Additionally, on October 8, 2021, Cook Medical issued a global, voluntary recall of the Transseptal Needle and the Transseptal Needle with Catheter due to reports of rust which could lead to increased inflammation.
-
July 2015
Cook Medical initiated a lot-specific voluntary recall of 2,239 lots of Beacon Tip Angiographic Catheters, including specific versions and lot numbers of the Torcon NB Advantage Beacon Tip Catheters (Catalog Prefix HNBR5.0), Royal Flush Plus Beacon Tip High-Flow Catheters (Catalog Prefix HNR4.0) and Slip-Cath Beacon Tip Catheters (Catalog Prefix SCBR5.0). A total of 95,167 devices globally were also subject to this recall.
-
October 2015
Cook Medical expanded the voluntary recall to include additional product lots of its following catheters: Torcon NB Advantage Beacon Tip Catheters, Royal Flush Plus Beacon Tip High-Flow Catheters, Slip-Cath Beacon Tip Hydrophilic Catheters and Shuttle Select Slip-Cath Catheters. Numbers of devices affected increased to 408,011 distributed throughout the U.S.
-
February 2016
The FDA identified the voluntary recall as a Class I recall, the most serious type of recall.
-
April 2016
Cook Medical expanded its voluntary recall to include all catheters with Beacon Tip technology. This recall included 4,146,309 catheters from all over the world.
Cook Medical’s Litigation History
Cook Medical is presently in the midst of litigation for two of its other malfunctioning devices: its pelvic repair system products and IVC filters. The manufacturer’s pelvic repair system, which is implanted in women suffering from stress urinary incontinence or pelvic organ prolapse, contributed to Cook Medical being named as one of five vaginal device manufacturers facing personal injury claims said to have occurred as a result of the defective product. The consolidated case is currently pending in the Southern District of West Virginia, MDL No. 2440.
Additionally, Cook’s IVC filters were found in a study to have high rates of perforation, piercing the wall of the vein in which they were implanted. They were also found to frequently move from their original positions. Over 100 resulting lawsuits were consolidated into a multidistrict litigation (MDL) court in 2014 where the case is now pending in the Southern District of Indiana, MDL No. 2570.
Potential Angiographic Catheter Lawsuits
Currently, Cook Medical is not facing any lawsuits regarding the malfunction of its Beacon Tip Angiographic Catheters. However, several law firms are presently seeking out consumers who may have a case against the company for harm caused by its defective catheters. Many law firms are still collecting client information and many consumers may still be presenting themselves as potential candidates with possible claims for damages as victims of serious injury or death resulting from the use of one of the affected catheters utilizing the Beacon Tip technology.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

