Mirena Side Effects
Mirena is a hormonal form of birth control. Some common side effects of the Mirena IUD include unscheduled (spotty) bleeding, missed periods, and abdominal or pelvic pain. Less common side effects include breast pain, benign ovarian cysts and, in rare cases, include severe pelvic pain or infections.
Common Mirena Side Effects
Mirena is a progesterone IUD that is considered a safe and effective form of birth control, however, it can cause side effects including cramps and mood swings. Compared to other hormonal birth control, the severity and risk of these side effects are relatively low. Most Mirena side effects last anywhere from a few hours to days after implanting the birth control device.
- Changes in menstruation: Many women choose Mirena because of its ability to lessen or eliminate period flow. During the first three to six months, periods may be lighter. Bleeding and spotting between periods, as well as missed periods (amenorrhea) may also occur. Heavy bleeding is rare.
- Tenderness and pain: Abdominal or pelvic pain, mild cramping, back pain, headaches and migraines may occur. More than 5% of women who used the Mirena IUD in clinical trials reported breast pain or tenderness.
- Skin Issues: Hormonal acne, itching, and rashes have all been reported. Changes in hair growth, including hair loss or gain, may also occur.
- Gastrointestinal issues: Bloating, nausea and vomiting are common, but typically do not last long. Changes in estrogen levels can also cause water retention, which can add to feeling bloated.
Copper IUDs don’t use hormones and are not linked to weight gain, but there have been suggestions that weight gain is a common side effect of Mirena. Because the Mirena device uses levonorgestrel, a progestin hormone, a small percentage of patients may experience weight gain, although this is typically less severe than with other contraceptives. Definitive evidence connecting Mirena to weight gain is lacking, however.
Rare and Serious Side Effects of Mirena
Rare side effects, such as migration or perforation of the uterus, are more serious to women’s health, and occur in fewer than 5% of users. These may require removal of Mirena.
The Mirena IUD releases a small amount of levonorgestrel directly into the uterus, but typically this does not affect breastmilk quality, production or how long you can breastfeed. If, however, your doctor inserts Mirena while you are breastfeeding, there is an increased risk of more serious side effects like perforation and IUD migration.
Mirena is not for everyone. If you have certain conditions, such as liver disease or cancer, or conditions of the uterus, such as fibroids or pelvic inflammatory disease, Mirena is not recommended. Talk to your doctor about the risks and whether Mirena is right for you.
Device Expulsion
In 2 to 3% of women using Mirena, the device moves out of position in the uterus. Expulsion impacts Mirena’s contraceptive effectiveness, increasing the risk of becoming pregnant.
If you feel the strings are lower than they should be and experience unusual cramping, you may have a partial expulsion. A full expulsion occurs when the IUD moves out of the uterus and into the vagina.
Perforation
Perforation, when the IUD pushes into the wall or muscle of the uterus, is very rare. It occurs in just one or two IUD insertions in a thousand.
When perforation does occur, it typically happens during the process of Mirena insertion. If your doctor sees a perforation, they will remove the IUD.
A recent study indicates that for women with an IUD placed in the year after giving birth, the risk of perforation increases nearly seven times if inserted between four days and six weeks postpartum. The risk was relatively lower if the IUD was inserted more than a year after delivery.
Migration or Displacement of Mirena IUD
Migration or displacement occurs when Mirena moves to another place in the body. This can happen after Mirena perforates the uterine wall and leaves the uterus.
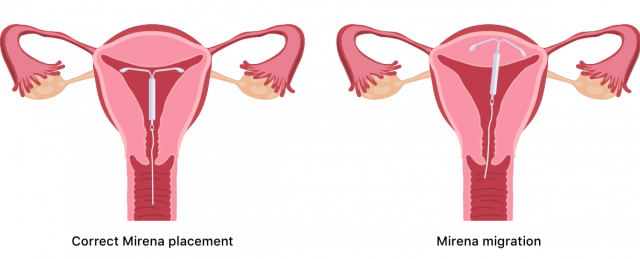
However, sometimes doctors don’t find evidence of perforation. It is also rare but possible for a piece of the IUD to break and migrate to another part of the pelvis.
Pelvic Inflammatory Disease and Infection with Mirena
An IUD slightly increases the chances of an infection of the uterus, which is also known as a pelvic inflammatory disease (PID). It is a potentially severe complication that typically arises because of the insertion process but can be treated with antibiotics.
If you have an undiagnosed sexually transmitted disease (STD) at the time your IUD is inserted, you are at higher risk of PID. Your doctor will test you for STDs before inserting Mirena.
Unexpected Pregnancy & Ectopic Pregnancy
The risk of getting pregnant while using Mirena is less than 1%. Symptoms of a regular pregnancy include nausea, fatigue, tender breasts and lack of periods.
If you do become pregnant while using Mirena, you are at a higher risk for an ectopic pregnancy. In an ectopic pregnancy the egg implants in a place outside the uterus, such as a fallopian tube.
- Sharp pain in the abdomen or pelvis
- Lower back pain
- Dizziness or fainting
- Vaginal bleeding
- Rectal pressure
- Bladder or bowel problems
Pregnancy complications with an IUD in place include preterm delivery, bacterial infection, low birth weight and pregnancy loss. Contact your health care provider if you experience any of these symptoms.
In Mirena lawsuits, women said the device migrated from its proper location and resulted in organ perforation and ectopic pregnancy.
Pseudotumor Cerebri
Hormonal contraception and IUDs have been linked to an increased risk of pseudotumor cerebri. This serious medical condition mimics the effects of a brain tumor and can cause migraines, vision loss and even permanent blindness.
Intracranial (brain) pressure characterizes pseudotumor cerebri. A build-up of cerebrospinal fluid causes the condition that is most commonly reported in women between the ages of 20 and 50.
Symptoms may include but are not limited to frequent headaches, ringing in the ears, dizziness, neck and shoulder pain, forgetfulness, or depression.
Longer-Term Side Effects of Mirena IUD
Bayer estimates that 20% of women who use Mirena will stop having their period after one year. Mirena is helpful in treating heavy periods and excessive menstrual bleeding. Most women return to a regular cycle within a few months of having Mirena removed.
Some studies have found that mental health related side effects may occur, but are generally temporary, lasting only a few weeks after removal. Other reports, however, have suggested symptoms may last for months.
- Ovarian cysts: Women using Mirena are more likely to develop benign ovarian cysts. Most ovarian cysts are harmless and painless and go away on their own. If they cause pain, however, they may be removed surgically.
- Depression and Anxiety: Emerging research has shown an increase in depression and anxiety stemming from levonorgestrel-releasing IUDs such as Mirena.
Side effects stemming from Mirena removal are sometimes called the “Mirena Crash.” Individuals respond to levonorgestrel differently.
Speak with your doctor if you develop cramping, migraines, severe pelvic pain, any symptoms of pseudotumor cerebri or any other unusual symptoms.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


