Abbott Flags Manufacturing Defect in FreeStyle Libre 3 Glucose Sensors
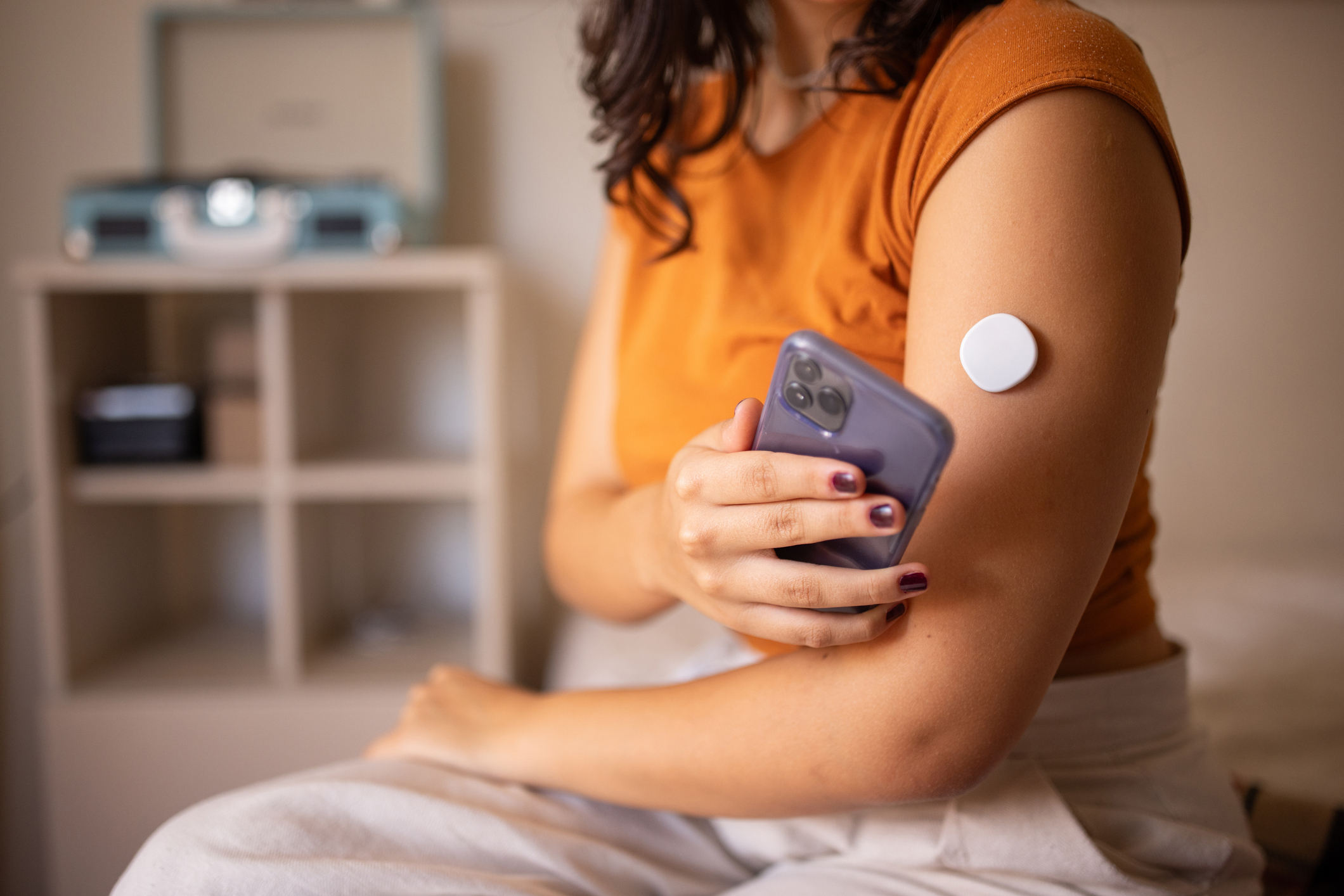
Millions of FreeStyle Libre 3 and Libre 3 Plus glucose sensors used by people with diabetes were flagged by the manufacturer as defective, linked to inaccurate glucose readings that could cause serious health complications.
The device maker, Abbott Laboratories, announced the defect in late November and issued a medical device correction for the sensors. A correction allows affected products to remain on the market while specific units are repaired, replaced or removed from use.
In this case, the company is replacing impacted sensors at no charge, and the defect was limited to a specific production line.
Abbott warned that inaccurate low glucose readings could affect treatment decisions made by people with diabetes, like delaying insulin doses or consuming carbs when unnecessary. If the issue is not detected, the company said it could increase the risk of serious injury or death.
According to Abbott, the manufacturing issue has since been resolved.
Scope of the Correction and Reported Adverse Events
About 3 million sensors were distributed in the U.S. from the impacted production line, though the company estimates roughly half have been used or have since expired.
Abbott received hundreds of reports of severe adverse events worldwide that were potentially linked to the inaccurate glucose readings, including seven deaths. At the time of the correction announcement, the company said no deaths were reported in the U.S., though 57 adverse events involved U.S. users.
Lawyers are now investigating lawsuits involving FreeStyle Libre devices.
Consumers who use FreeStyle Libre devices can check www.FreeStyleCheck.com to determine whether their sensor is part of the correction. They can also request a replacement through the site.
Accuracy Concerns Regarding Glucose Monitoring
Continuous glucose monitors, or CGMs, are medical devices that allow people with diabetes to track glucose levels without fingerstick testing. These devices help patients and their doctors monitor how well their treatment plan is working and make decisions about insulin use or other treatment options.
CGMs, like FreeStyle Libre 3, use a small sensor placed under the skin to continuously measure glucose levels and transmit them in real time to an app, a receiver or an insulin pump, depending on the system and the type of diabetes being treated.
Because many users rely on CGMs to monitor blood sugar, the accuracy and reliability of glucose readings are important for patient safety.




