Truvada
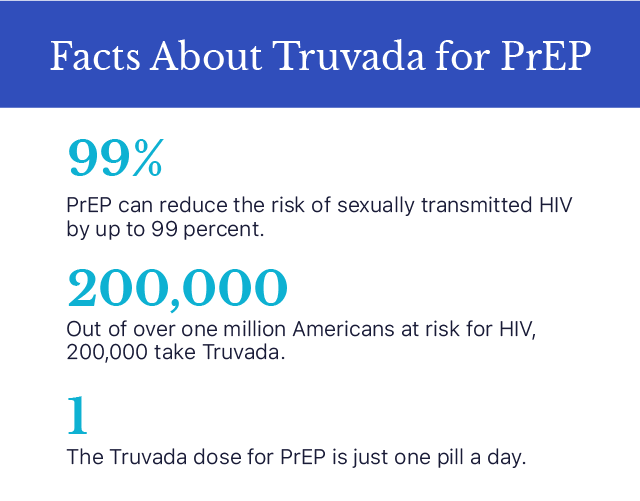
Truvada — emtricitabine and tenofovir disoproxil fumarate — is a prescription drug used as pre-exposure prophylaxis, or PrEP, to prevent HIV. It also treats HIV in combination with other antiviral drugs. The CDC says Truvada when taken daily is up to 99 percent effective when used as PrEP. Common side effects when using Truvada for PrEP include headache, abdominal pain, and weight loss. More serious and rare side effects include kidney, liver, and bone problems.
- Medically reviewed by Katherine V. Sarna, Pharm.D., BCPS
- Last update: April 16, 2025
The U.S. Food and Drug Administration first approved Gilead Sciences’ Truvada in 2004. The FDA approved it for pre-exposure prophylaxis, or PrEP, to prevent HIV infection in 2012.
One of the drug’s main active ingredients, tenofovir disoproxil fumarate, or TDF, first appeared in Gilead’s HIV medication, Viread, in 2001. Since then, the drugmaker has manufactured several TDF drugs in combination with other anti-HIV medications. In addition to Truvada, Gilead’s TDF-containing drugs include Viread, Atripla, Complera, and Stribild.
“Approximately 200,000 of the estimated 1.1 million Americans who are at risk for HIV currently receive Truvada for PrEP,” Gilead said in a 2019 press release.
According to the company’s 2021 annual report, it achieved record sales for its HIV products in 2018, and sales of HIV treatments accounted for 67 percent of its total product sales.
Between 2004 and 2015, Gilead’s estimated profit from Truvada was $36.2 billion, according to court documents.
How Truvada Works
Truvada’s two active ingredients, emtricitabine and TDF, work together to block an enzyme called HIV reverse transcriptase. By blocking the enzyme, the drug prevents HIV from multiplying. It may also reduce the amount of HIV in the body.
The drug is not a cure for HIV/AIDS, but it can help infected people live longer. It also reduces the risk of transmitting the disease to others.
What is Pre-Exposure Prophylaxis?
PrEP is an HIV prevention approach where people without HIV use anti-HIV medications to decrease their chances of getting infected with the virus. Having the medicine in the body makes it harder for HIV to spread throughout the body and establish an infection.
- You are HIV-negative, but in the past six months had a sexual relationship with a partner who is HIV-positive
- You are HIV-negative and had an STD or sex without consistent use of condoms in the past six months
- You are HIV-negative and inject drugs, and have an injection partner that is HIV-positive or share needles, syringes, or other equipment to inject drugs
PrEP is different from post-exposure prophylaxis, or PEP. People who use PEP take medications within 72 hours of coming into contact with HIV to reduce infection risk. PEP treatment lasts for a month.
How Effective is Truvada?
Truvada has been proven to reduce the risk of getting HIV through sex and through intravenous drug use, according to clinical trial data presented on Truvada’s website.
However, the study data presented by Gilead is a small sample. Out of 1,251 men who have sex with men and transgender women who have sex with men taking Truvada, Gilead sampled 10 percent. This group of 125 people had a 92 percent decreased chance of HIV infection.
Gilead found similar results in a group of 158 heterosexual people where one partner had HIV and the other did not.
The CDC says Truvada is up to 99 percent effective when taken daily. The 2012 iPrEx clinical trial by researcher Peter L. Anderson and colleagues found that the effectiveness of Truvada goes down when not taken daily.
- 99 percent estimated effectiveness for people who take 7 pills per week
- 96 percent estimated effectiveness for people who take 4 pills per week
- 76 percent estimated effectiveness for people who take 2 pills per week
How Much Does It Cost?
The average cost of Truvada is $1,600 to $2,000 for a one-month supply. The out-of-pocket cost for Truvada varies by insurance plan and available financial assistance programs.
Gilead offers programs to help people get Truvada for as little as $0 co-pay. Uninsured people may also get PrEP medication free from manufacturer-sponsored programs.
On May 9, 2019, Gilead announced it would donate up to 2.4 million bottles of Truvada each year to the Centers for Disease Control and Prevention.
The donation is for uninsured Americans at risk of HIV and will last until 2030. Gilead plans to transition the donation from Truvada to Descovy, which was approved for PrEP in October 2019.
Generic Available in 2020
In Gilead’s quarterly report ending March 31, 2019, the company announced that it reached a patent settlement agreement allowing Teva Pharmaceuticals to launch a generic version of Truvada on September 30, 2020.
Important Safety Information
It’s important to get tested for HIV before taking Truvada for PrEP. Only HIV-negative people may take the drug for PrEP. Health care providers will continue to test people for HIV while they are on Truvada to make sure they remain negative.
The HIV medication does not protect against all sexually transmitted diseases. People who take the drug should continue to practice safe sex because Truvada alone might not prevent HIV transmission.
The most common Truvada side effects in patients who take it for PrEP are mild, according to the drug’s insert. Clinical studies showed the three most common side effects in PrEP patients were headache, abdominal pain, and weight loss.
HIV-infected patients using Truvada to treat HIV suffered more side effects.
- Diarrhea
- Nausea
- Vomiting
- Fatigue
- Headache
- Dizziness
- Depression
- Insomnia
- Abnormal dreams
- Rash
- Upper respiratory tract infections
- Nasopharyngitis
- Sinusitis
More serious side effects include kidney or liver problems, decreases in bone mineral density, lactic acidosis and immune reconstitution syndrome — an inflammatory response that can cause worsening infections in people who take antiretroviral drugs.
Truvada has a black box warning for acute exacerbations of hepatitis B virus (HBV) in patients who stop taking the drug. Health providers should monitor patients with HBV who stop treatment. Another black box warning for Truvada states that the drug should only be used for PrEP in patients who are HIV-negative.
How to Take Emtricitabine and TDF
People take one Truvada pill for PrEP once a day with or without food. The dose should be taken at the same time each day. It’s important not to skip doses, as skipping doses may leave people open to HIV infection.
It takes at least seven days after starting PrEP to reach the highest levels of protection from HIV.
Prior to taking Truvada, doctors must test patients for HBV and HIV infection. Patients with kidney problems may take lower doses.
Strengths
Tablets: 200 mg/300 mg, 167 mg/250 mg, 133 mg/200 mg, and 100 mg/150 mg of emtricitabine and tenofovir disoproxil fumarate, respectively.
- Recommended dosage in HIV-1 uninfected adults and adolescents who weigh at least 77 lbs: One tablet by mouth once daily with or without food.
- HIV-uninfected people should not take Truvada if they have kidney problems (creatinine clearance [CrCl] is below 60 mL/min).
- Recommended dosage in adults and pediatric patients who weigh at least 77 lbs: One tablet containing 200 mg of emtricitabine and 300 mg of TDF once daily by mouth with or without food
- In pediatric patients who weigh at least 37.5 lbs: One low-strength tablet (100 mg/150 mg, 133 mg/200 mg, or 167 mg/250 mg based on body weight) by mouth once daily with or without food
- If CrCl is 30 to 49 mL/min: One tablet every 48 hours
- Patients with a CrCl is below 30 mL/min or those on hemodialysis should not take Truvada.
Drug Interactions
Some drugs can interact with Truvada and increase or decrease its strength. They can cause additional side effects or prevent Truvada from working effectively.
- Didanosine
- Taking Truvada with didanosine increases didanosine levels in the body. This may cause symptoms of didanosine toxicity such as pancreatitis or neuropathy.
- HIV-1 protease inhibitors
- When taken with atazanavir and ritonavir, darunavir and ritonavir, or lopinavir and ritonavir, the levels of tenofovir in the body increase. Therefore, patients should be monitored for evidence of tenofovir toxicity.
- Atazanavir
- Taking Truvada with atazanavir decreases atazanavir concentrations. Atazanavir should be given with ritonavir in patients who also take Truvada.
- Hepatitis C Antiviral Agents
- Drugs such as Vosevi, Epclusa, and Harvoni increase concentrations of tenofovir and may cause increased adverse reactions.
- Drugs Affecting Renal Function
- Because the kidneys eliminate Truvada, drugs that reduce kidney function can increase the concentration of the drug in the blood. Examples include: cidofovir, acyclovir, valacyclovir, ganciclovir, valganciclovir, gentamicin, and high-dose or multiple NSAIDs (aspirin, ibuprofen, etc). Don’t use Truvada with Hespera (ie, adefovir).
This isn’t a complete list of all drug interactions. Make sure you tell your health care provider about all medication, vitamins and herbal supplements you take.
Descovy vs. Truvada
Until the FDA approved Descovy (emtricitabine and tenofovir alafenamide) for PrEP in October 2019, Truvada was the only PrEP drug on the market. But Descovy isn’t approved for PrEP in cisgender women.
Initial studies also show that Descovy is as effective as Truvada and is less harmful to bones and kidneys than Truvada.
| Category | Truvada | Descovy |
| Active Ingredient | emtricitabine and tenofovir disoproxil fumarate (TDF) | emtricitabine and tenofovir alafenamide (TAF) |
| What Does it Treat? | HIV in adults and pediatric patients who weigh at least 77 pounds in combination with other antiretroviral drugs | HIV in adults and pediatric patients who weigh at least 77 pounds in combination with other antiretroviral drugs |
| Approved for PrEP | Yes. Approved for adults and adolescents. | Yes. But only approved for adult and adolescent men and transgender women. Descovy has not been tested or approved for use by cisgender women. |
| Instructions | One tablet orally once a day, with or without food. | One tablet orally once a day, with or without food. |
| Available Strengths | emtricitabine 100 mg to 200 mg combined with TDF 150 mg to 300 mg | emtricitabine 200 mg combined with TAF 25 mg |
| Cost | $1,600 to $2,000 a month | About $1,800 a month |
| Common Side Effects for PrEP patients | Headache, abdominal pain and weight loss | Diarrhea, nausea, headache, fatigue, and stomach pain |
Kidney and Bone Damage Lawsuits
Truvada and its active ingredient TDF have made billions for Gilead, but the drugs have had their share of controversy.
As far back as 2000, the FDA has warned Gilead about downplaying the risks of its HIV medications containing TDF, starting with Viread, The Los Angeles Times reported.
While downplaying the risks of kidney and bone damage, it exaggerated the benefits. A Gilead salesperson even told an FDA official that Viread was “a miracle drug.”
In 2016, AIDS Healthcare Foundation (AHF) accused Gilead of intentionally delaying the release of a stronger, safer medication called tenofovir alafenamide, or TAF — one of the active ingredients in Descovy — to maximize profits from TDF.
In 2018, AHF filed a personal injury lawsuit on behalf of two men injured by Truvada and other TDF drugs as well as a class action against Gilead.
People who suffered kidney disease or bone density loss and fractures filed hundreds of Truvada lawsuits claiming the drug maker failed to warn the public about the risk. They also claim the delay in releasing TAF caused patients to suffer from serious side effects unnecessarily.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


