Paragard Lawsuit
Paragard lawsuits claim that the Paragard IUD can break upon removal and that the manufacturer failed to adequately warn the public. Claims from all over the country have been transferred to federal multidistrict litigation in Georgia to streamline proceedings.
- Legally reviewed by Trent B. Miracle, Esquire
- Last update: July 9, 2025
Latest Paragard Lawsuit Updates
Litigation involving the Paragard intrauterine copper contraceptive device is ongoing. Our legal partners are actively accepting new cases on behalf of women who experienced pain, infertility and other injuries from broken Paragard IUD pieces embedded in their organs.
As of July 2025, there were 3,474 pending lawsuits and a total of 3,717 cases filed against the company in federal court in Georgia under MDL number 2974.
The first bellwether trial is expected to begin in January 2026.
From our legal research through court documents and talking to our legal partners, we’ve compiled a timeline of the biggest developments in this litigation below.
-
May 2025:
The first bellwether trial case has been selected in the Paragard MDL. After reviewing possible options submitted by both sides, the judge overseeing the litigation has selected a case that was suggested by the defendants. The first trial is slated to begin in January 2026.
-
February 2025:
In a blow to plaintiffs, the judge overseeing the Paragard lawsuits pending in federal court opted to dismiss claims from several states over statute of limitations issues. The issue in question was pinpointing when plaintiffs’ injuries occurred and when exactly the clock starts on filing a claim. The court essentially determined that injuries took place while the device was still in plaintiffs’ bodies. “It would defy logic to find that a broken Paragard may not have inflicted medically ascertainable injuries until after it had already been removed from the woman’s body,” the judge stated.
Also in February, the start of the Paragard bellwether trials was once again delayed. The first trial was now set to begin Jan. 12, 2026. -
October 2024:
The court scheduled two bellwether trials to begin Dec. 1, 2025 and Feb. 2, 2026, pending availability of defendants' counsel.
-
August 2024:
No new developments happened in August in the multidistrict litigation. In past months, Judge Leigh Martin May replaced some members of the Plaintiffs’ Steering Committee and discovery continued to move forward slowly, according to our review of the MDL docket.
-
July 2024:
The next status conference was scheduled for the end of July.
-
December 2023:
Several cases joined the MDL and conditional transfer orders were filed.
-
September 2023:
Judge Leigh Martin May outlined the protocols for depositions for bellwether pool plaintiffs in October 2023. The first trial was expected to be on or before Oct. 28, 2024, according to the judge’s order.
-
February 2023:
The judge outlined the process for selecting 10 cases to be the first group of bellwether test trials to go to trial in 2024.
-
January 2023:
Judge M. Gino Brogdon Sr. (retired) was selected to serve as a settlement mediator. We viewed the progress as a sign parties were negotiating a global Paragard settlement and expected talks to take place throughout 2023.
Paragard bellwether trials are called test trials because the outcome of these cases will help lawyers on both sides better evaluate a global settlement value.
In some cases, defendants will settle a case ahead of trial to avoid potentially paying more damages and risking a jury verdict.
Why Are Women Filing Paragard IUD Lawsuits?
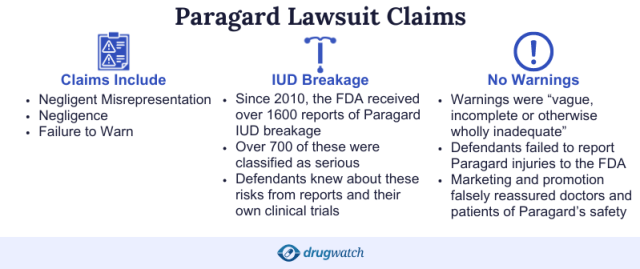
The Paragard IUD lawsuits claim a design flaw led to the IUD device breaking during removal. This meant that part of the device could remain lodged in the uterus or other internal organs, causing injuries.
The women claim that the manufacturers failed to warn doctors and patients of the risks. As a result, plaintiffs suffered several debilitating effects from undergoing Paragard IUD removal. Some women required surgery as a result.
“This litigation involves allegations that the Paragard intrauterine device (IUD) has a propensity to break upon removal, causing complications and injuries, including surgeries to remove the broken piece of the device, infertility, and pain,” according to the Northern District of Georgia’s MDL page.
- IUD has manufacturing and design defects.
- Labeling doesn’t adequately warn about breakage risk.
- The manufacturers, CooperSurgical and Teva Pharmaceuticals, were negligent.
In December 2020, the Judicial Panel on Multidistrict Litigation consolidated dozens of lawsuits across the nation in the Northern District of Georgia under Judge Leigh Martin May.
The first bellwether was scheduled for January 2024, then October 2024. Toward the end of 2024, we learned that two trials were set for December 2025 and February 2026. As of March 2025, the first bellwether trial was expected to begin in January 2026.
“This litigation involves allegations that the Paragard intrauterine device (IUD) has a propensity to break upon removal, causing complications and injuries, including surgeries to remove the broken piece of the device, infertility, and pain.”
Am I Eligible To File a Paragard Lawsuit?
According to our legal partners, you may qualify to file a Paragard IUD lawsuit if your Paragard IUD broke upon removal, and you experienced serious injury or Paragard side effects.
Women who filed lawsuits claim that Paragard broke during removal, leaving pieces of the IUD in their bodies. Some women required surgery to remove the device and treat complications.
According to CooperSurgical’s website, “Paragard removal is nonsurgical and done by a health care provider during a routine office visit in just a few minutes.” The arms of the device should fold up when removed, according to the instructions for removal included in the prescribing information.
- IUD pieces breaking and getting lodged in organs
- IUD pieces that cannot be removed from the body
- IUD pieces left in the body, causing infections and allergic reactions
- IUDs shifting position
- Pain
- Infection
- Perforation of the cervix or uterus
- Infertility
- Need for surgical intervention such as laparoscopy or laparotomy and hysterectomy
If you experienced an injury because of Paragard IUD removal, you may qualify to receive compensation for pain and suffering, medical bills or lost wages. Before moving ahead with litigation, it is important to speak to a lawyer to review your options and the specifics of your case.
Examples of Paragard Lawsuits
Paragard manufacturer CooperSurgical continues to make claims supporting the device’s safety and benefits. However, several women have already filed lawsuits after suffering injuries upon removal of their IUD.
Medical researchers investigating IUD side effects have also recommended improved protocols when removing Paragard IUDs.
Georgia Bowers v. Teva et al.
Georgia Bowers filed her suit against Teva and CooperSurgical in September 2020. She received her Paragard in January 2017. In September 2017, she visited her doctor to have the IUD removed.
An ultrasound revealed the device was incorrectly positioned. Bowers’ doctor followed instructions for removing the IUD, but only part of it came out. One arm was missing. Bowers’ doctor tried to remove the broken piece by colposcopy but failed.
“Defendants had knowledge that there was a significant increased risk of adverse events associated with Paragard IUD, including arm breakage, and despite this knowledge … Defendants continued to manufacture, market, distribute, sell and profit from sales of Paragard IUD.”
“At all relevant times, the Teva Defendants had knowledge that there was a significant increased risk of adverse events associated with Paragard IUD, including arm breakage, and despite this knowledge the Teva Defendants continued to manufacture, market, distribute, sell and profit from sales of Paragard IUD,” Bowers’ lawsuit said.
Carley Tredway v. Teva et al.
Carley Tredway filed her suit against Teva and CooperSurgical in September 2020. Tredway received her Paragard in 2008. In 2018, she went to get her Paragard removed.
“Plaintiff and her doctors were provided with no warning from the Defendants of the risk of Paragard IUD failure and injury, nor were Plaintiff and her doctors provided with adequate warning of the risk of removal of ParaGard IUD.”
Her doctor followed the instructions to remove the IUD, but one arm remained in the uterus upon removal. Tredway’s doctor removed the remaining arm via hysteroscopy a month later.
“Prior to her procedures, Plaintiff and her doctors were provided with no warning from the Defendants of the risk of Paragard IUD failure and injury, nor were Plaintiff and her doctors provided with adequate warning of the risk of removal of ParaGard IUD,” Tredway’s complaint said. “This information was known or knowable to the Defendants.”
Paragard Lawsuit Settlement Amounts
The Paragard litigation is in progress and as of July 2025, there has been no global settlement. If an out-of-court settlement isn’t reached, the first trials may begin in 2025. The result of these first trial cases will likely influence possible settlement amounts.
Prior IUD birth control cases like the Mirena IUD settlement can provide some indication of lawsuit settlement amounts, but this is only a guess, and you shouldn’t base any potential settlement amounts on this figure.
In August 2017, Bayer offered $12.2 million to settle 4,600 lawsuits after women successfully sued the manufacturer for claims that their IUD moved around in the body and punctured or injured organs.
If you have questions about any potential settlement amounts, make sure you speak to your lawyer directly.
Settlements can sometimes be reached before a trial begins or once it is underway. Monetary settlements for damages can vary significantly based on the details of an individual case. Settlement talks generally start once the first few test cases go to trial and juries award verdicts.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.





