Bard PowerPort: Device Failures, Injuries and Safety Concerns
A Bard PowerPort is a device used to administer treatments like chemotherapy or fluids through a vein. However, lawsuits say PowerPorts broke or failed, causing serious injuries. These problems have led to lawsuits against the device’s manufacturer.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
This site is an amazing resource! I found the detailed information about the pro...
- Legally reviewed by Brendan A. Smith, Esquire
- Last update: February 25, 2026
- Est. Read Time: 6 min read
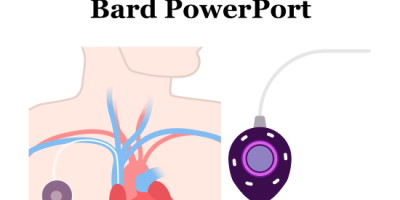
The Bard PowerPort is a small, implanted medical device that sits under the skin and connects to a thin, flexible plastic tube (catheter) that runs to a large vein. Medical providers use it for long‑term intravenous (IV) access for medications, fluids and blood draws.
Bard designed these catheters with ChronoFlex, a material made up of polyurethane and barium sulfate. According to Bard PowerPort lawsuits, this material is prone to cracking and fracturing, which can cause injuries such as blood clots, cardiac injuries, infections and damage to blood vessels, leading to hospitalizations and even death.
As of February 2026, more than 2,832 Bard PowerPort lawsuits are consolidated in a federal multidistrict litigation (MDL).
The first federal Bard PowerPort bellwether trial for the case of Robert Cook is set to begin on April 21, 2026. This case will help test core evidence about alleged device defects and injuries before a jury.
What Is a Bard PowerPort?
A Bard PowerPort is a small medical device placed under the skin that helps doctors access veins more easily without needing to place a new IV into the skin each time.
PowerPorts were manufactured by Bard, formerly known as C.R. Bard, a medical device manufacturer that is part of Becton, Dickinson and Company.
Common Medical Uses
Common uses of the Bard PowerPort catheter involve delivering liquids or medications to patients. Medical providers can also take blood samples.
When used with a special needle, it can handle strong injections, including contrast dye used in medical scans.
- Blood transfusions
- Chemotherapy
- IV fluids
- Nutrition support
Since the port is placed under your skin, it can remain in place for years, making it easier for people who require repeated treatment or tests.
Bard obtained FDA clearance to sell one of its PowerPorts with ChronoFlex in 2006. Unfortunately, PowerPort failures have led to thousands of lawsuits claiming design defects resulted in complications and severe injuries.
How Bard PowerPorts Work And Why They Fail
Doctors place the Bard PowerPort completely under the skin, usually in the upper chest. The port connects to a flexible catheter that threads into a large central vein near the heart.
Medical providers deliver medications or other fluids to patients by injecting them into the port. They can also insert a needle into the port to draw blood.
However, thousands of patients have filed lawsuits against Bard and its parent company, Becton, Dickinson and Company (BD), claiming that Bard manufactured the catheters with defective material, making them prone to failure.
The ChronoFlex Material Defect
Lawsuits say that Bard PowerPort catheters use a material called ChronoFlex that is defective because it can break down, leading to cracks in the catheter. ChronoFlex is made of polyurethane and barium sulfate.
Lawsuits allege excessive barium sulfate weakens the catheters over time and can cause cracks, fissures, divots and pitting on their surfaces.
Bard PowerPort Problems and Device Failures
Bard PowerPort complications and device failures range from catheter fracture to serious infection risks.
“Many of our clients suffered serious complications after their Bard PowerPort device fractured or migrated,” attorney Moshe Horn of Simmons Hanly Conroy told Drugwatch.
Catheter Fracture and Breakage
Fractures and breaks occur when pieces of the catheter fail. According to a study in the Journal of the Mechanical Behavior of Biomedical Materials, loss of barium sulfate particles can cause notches in the catheter and lead to fractures.
Device Migration
A catheter might move from its original position, causing discomfort and possibly requiring surgery to fix. In one study, researchers found that the Bard Powerport migration rate was 6.7%. For patients who experienced port migration, most incidents occurred within 30 days of receiving the port.
“Many of our clients suffered serious complications after their Bard PowerPort device fractured or migrated."
Infection Risk
Many catheters can last for years, but defective Powerport catheters can break down and develop cracks that allow bacteria to collect, increasing the risk of infection. This may require the removal of the catheter and negatively impact treatment.
Serious Injuries From PowerPort Failures
Serious injuries from PowerPort failures include blood clots, cardiac complications, infections and organ damage. If you suffer any of these injuries, make sure to seek medical attention right away.
Blood Clots and Pulmonary Embolism
Blood clots that form in the vein, also called deep vein thrombosis (DVT), can form around the device and potentially travel to the lungs, resulting in a pulmonary embolism. In general, studies say the rate of DVT is 5% to 40% in cancer patients who used port catheters.
The FDA’s MAUDE database includes 438 adverse event reports between Dec. 1, 2025, and Feb. 19, 2026. Of those reports, there were 111 blood clots and 17 pulmonary embolisms.
Cardiac Complications
Fractured Powerport catheters may lead to heart problems such as ruptures, irregular heartbeats, clots or blood infections. Fluid accumulation around the heart (cardiac tamponade) can create pressure, preventing the heart from pumping blood effectively. If not treated promptly, it can lead to organ failure, shock or death.
Infections and Sepsis
Some patients have had infections at the site of the medical device or in their blood (sepsis), which may require removing the device and using strong antibiotics. In one study, 50% of patients who developed PowerPort complications had to have the catheter removed due to infection.
Lung Puncture
Small fragments of a fractured catheter may lodge in the heart, but they can also damage other organs, such as the heart, when they travel through the blood. It may lead to internal bleeding.
Emergency Warning Signs: Pulmonary Embolism
If you have a Bard PowerPort, it’s important to recognize the symptoms of a pulmonary embolism. This is a serious condition that can affect your lungs and the symptoms can vary.
Pulmonary embolisms are life-threatening and require immediate medical attention.
- Chest pain
- Clammy or discolored skin
- Coughing up blood or blood-streaked mucus
- Dizziness or lightheadedness
- Excessive sweating
- Fainting
- Fast or irregular heartbeat
- Fever
- Leg pain or swelling, especially in the lower back area of your leg
- Shortness of breath
If you or someone you know has these symptoms, seek medical attention immediately. Have someone drive you to an emergency room or call 911.
FDA Recalls, Safety Reports and Regulatory Oversight
The FDA has received hundreds of adverse event reports related to the Bard PowerPort. The FDA’s MAUDE database includes 438 reports between Dec. 1, 2025, and Feb. 19, 2026 alone.
Despite this, there have been no Bard PowerPort or FDA recalls due to the device deteriorating over time. Bard said it didn’t find any new safety risks in a 2023 statement after the MDL was announced.
However, Bard recalled 178 PowerPort devices in October 2019 due to another issue. Some devices may have been shipped with a connection designed for a different type of catheter, which could prolong the implantation surgery.
510(k) Clearance Timeline
The FDA’s 510(k) process allows a new device to be cleared if it’s shown to be as safe and effective as one already on the market, and this is how Bard PowerPort devices made it onto the market. The device doesn’t have to be identical, but it must function the same way without raising new safety concerns.
Approximately 99% of medical devices have been approved through the 510(k) pathway since the 1970s. Critics argue that this process may allow risky devices to reach the market because it lacks specificity and lets companies cite currently marketed devices in a potentially unsafe way.
| Date | Regulatory Action/Device | Key Details |
|---|---|---|
| July 14, 2006 | 510(k) clearance for PowerPort Titanium Port with 8 Fr ChronoFlex catheter (K060812) | FDA finds the PowerPort implanted titanium port with 8 Fr ChronoFlex catheter substantially equivalent to prior devices, classified under product code LJT. |
| November 20, 2015 | 510(k) clearance for power-injectable implantable ports with ChronoFlex catheters | FDA clears Bard’s ChronoFlex polyurethane catheter ports, finding them substantially similar to earlier devices. |
| July 8, 2019 | 510(k) clearance for updated PowerPort models | Updated PowerPort designs receive clearance, with FDA again confirming substantial equivalence to prior models. |
| December 8, 2023 | 510(k) clearance for PowerPort ClearVUE Slim ECG Enabled Implantable Port | ECG-enabled ClearVUE Slim and related models are cleared, reflecting ongoing FDA review and oversight. |
| October 31, 2024 | 510(k) clearance for PowerPort ClearVUE Slim Implantable Ports | Additional ClearVUE Slim ports obtain 510(k) clearance, and the devices retain their Class II status. |
PowerPort Lawsuits and Legal Action
PowerPort lawsuits and legal actions stem from plaintiffs’ claims that Bard’s PowerPort catheter designs are defective. According to lawsuits, excessive levels of barium sulfate in the tubing weaken the catheters, making them more likely to become damaged.
People who used the catheters and suffered a catheter fracture, catheter migration, infection or blood clots (thrombosis) that required catheter removal may qualify to file a lawsuit. For more information, you can read about filing a claim on our Bard PowerPort lawsuit page.
What the Lawsuits Allege
One of the main allegations in PowerPort lawsuits is that Bard knew the PowerPort catheters were defective and could cause injuries but failed to warn patients and doctors of the risk.
Judges consolidated PowerPort lawsuits from across the country into multidistrict litigation (MDL) 3081 in Arizona in August 2023.
MDL Status and Bellwether Trials
As of February 2026, more than 2,832 Bard PowerPort lawsuits are consolidated in MDL 3081.
The most recent order from November 2025 shows the updated test trial (bellwether) schedule, with the first trial scheduled for April 2026.
| Case | Start Date | End Date |
|---|---|---|
| Robert Cook, 23-cv-01975 | April 21, 2026 | May 8, 2026 |
| Wanda Miller, 24-cv-00621 | July 7, 2026 | July 24, 2026 |
| May Lattanzio, 24-cv-00680 | August 18, 2026 | September 4, 2026 |
| Kimberly Divelbliss, 23-cv-01627 | October 13, 2026 | October 30, 2026 |
| Judy Hicks, 23-cv-01703 | December 1, 2026 | December 18, 2026 |
| Lloyd Sorensen, 23-cv-02557 | February 2, 2027 | February 19, 2027 |
Frequently Asked Questions About Bard PowerPort
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.