NuVasive MAGEC System Lawsuits
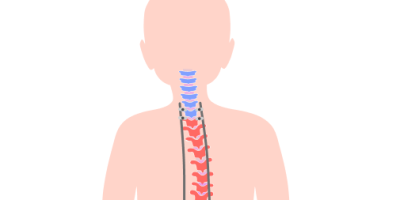
NuVasive recalled its MAGEC system after reports of endcaps detaching from implanted rods. Injuries include possible bone abnormalities, back pain and local tissue reactions.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Cassandra L. Sundblad, Esquire
- Last update: May 7, 2025
- Est. Read Time: 2 min read
Why Did People File Lawsuits Against NuVasive?
Caregivers and parents filed lawsuits against NuVasive because the endcaps of the MAGEC rods could detach and cause health problems.
NuVasive recalled the MAGEC system in February 2020 after it received reports of a mechanical component failure in which the endcaps were separating from the rods of the device.
In July 2020, the Food and Drug Administration cleared a new version of the MAGEC X rod that was intended to help with the endcap problem, but the agency began receiving reports of tissue reactions potentially related to endcap separation in early 2021.
The FDA raised concerns in a safety communication on July 15, 2021, that internal components exposed by the detached cap may be incompatible with the body. NuVasive is still investigating these potential biocompatibility issues.
As of March 2026, there have been no important developments in this litigation. Drugwatch’s legal partners are currently not accepting NuVasive MAGEC lawsuit cases.
MAGEC Rod Complications
Several studies have documented problems with MAGEC devices over the years. Reported complications and MAGEC system side effects range from device failure to inflammatory reactions from metal particles released into the tissues around the implant.
In these studies, complication rates ranged from 18% to 57%. A number of patients had to undergo additional unplanned surgeries to deal with device complications.
One 2019 study published in Asian Spine Journal found magnetically controlled growing rods were effective at treating pediatric scoliosis, but nearly half of the patients still developed complications or required unplanned operations.
- Rod breakage
- Fixation failure
- Fractured drive pin
- Infection
- Tissue death (necrosis)
- Metal particles released from wear
- Screw breakage
- Spinal deformities
- Autofusion of the spine
Which MAGEC Devices Have Been Recalled?
NuVasive issued a recall for its MAGEC system in February 2020. The company placed a global shipping hold on the devices in April 2021 because of biocompatibility concerns, and then it removed the hold in July 2021.
Currently, only the MAGEC X rods (MAGEC 2b) that were modified to lessen problems with endcap separation are available to American patients. U.S. labeling on the device now includes information about potential risks.
- MAGEC Spinal Bracing and Distraction System
- MAGEC 2 Spinal Bracing and Distraction System
- MAGEC System
- MAGEC System Model X device
- MAGEC System Model X rod
- MAGEC System Rods
The FDA continues to receive reports of tissue problems related to endcap separation even after the rods were redesigned. However, the agency determined the benefits of the MAGEC device currently outweigh the potential risks of biocompatibility and tissue reactions.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.