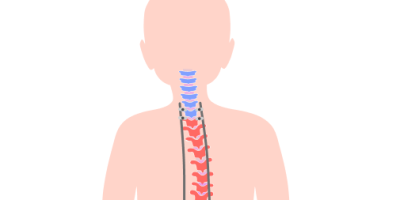
NuVasive MAGEC System
The NuVasive MAGEC system is a spinal implant that helps straighten the spine in children with scoliosis. Complications of the MAGEC system include device failure, rod fracture and unplanned surgeries. NuVasive issued a recall in February 2020 because implanted device endcaps could separate.
- Last update: March 7, 2025
What Is NuVasive’s MAGEC System?
NuVasive’s MAGEC system is a medical device that treats children with early onset scoliosis (EOS). Children with EOS have a spine that grows in curves or twists instead of straight. EOS affects kids before age 10.
The FDA approved the system in 2017 for patients with progressive or severe EOS who have failed nonoperative treatment.
MAGEC stands for MAGnetic Expansion Control. The MAGEC system is made up of adjustable growing rods controlled by magnets and an external remote control. A surgeon implants the growing rods into a child’s back to help straighten the spine and enable it to stay straight as the child grows.
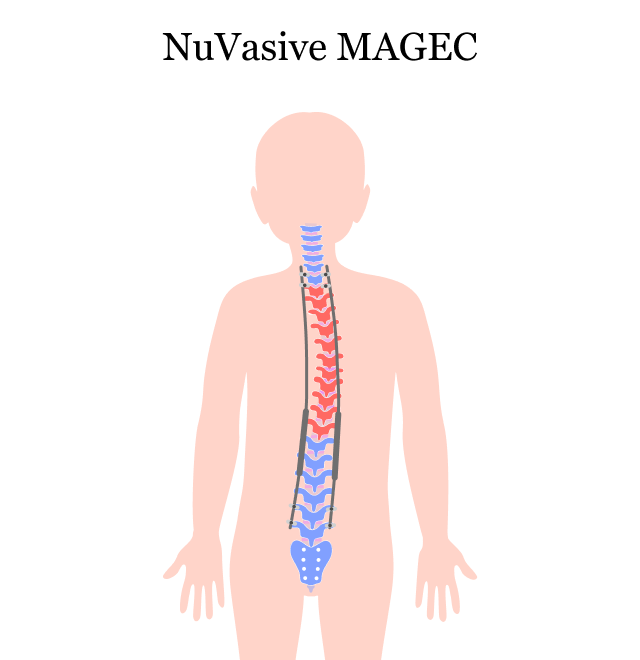
How MAGEC Treats Early Onset Scoliosis
Doctors have been using growing rods to treat EOS for several years. Surgeons implant two growing rods on either side of the spine. As children grow, the rods have to be lengthened every six months to facilitate spinal growth and straightening.
This process of lengthening and stretching the spine with implants is called distraction. Children implanted with traditional growing rods have to have distraction surgery every six months on average to adjust the rods as they grow.
With the MAGEC system, doctors implant special titanium growing rods into a child’s back once. This allows doctors to gradually stretch the spine by using a remote control and powerful magnets, without multiple invasive surgeries. This noninvasive distraction technique is an outpatient procedure that is typically quick and painless.
Potential Side Effects of the MAGEC System
There aren’t enough studies to determine how often MAGEC system side effects occur. But one 2020 study in the Journal of Pediatric Orthopaedics reported 10 out of 39 study participants implanted with the MAGEC system suffered implant-related complications.
A 2022 study in the Journal of Orthopaedic Surgery and Research found that 25% of patients who received magnetically controlled growing rods developed complications, including spinal alignment failure, decreased wound healing, pulmonary complications, back pain and fracture.
Another 2020 study in Spine Surgery and Related Research reported the system has a high failure rate compared to traditional rods. This may lead to unplanned revision surgery to replace the implant.
Researchers observed that the devices generate titanium debris which can cause metallosis, an inflammatory reaction that causes swelling, pain and tissue death around the implant.
Status of NuVasive’s MAGEC Recall
In February 2020, NuVasive recalled the MAGEC system because the endcap was separating from the ends of the rods. The danger of endcap separation is that a patient’s tissues could be exposed to components in the device which may not be biocompatible and may cause adverse reactions in the body.
In April 2021, NuVasive placed all MAGEC devices on a global hold until it could evaluate the biocompatibility testing concerns. The FDA approved a new version of the MAGEC X rod to address the issues of endcap separation. In July 2021, the hold was lifted and the newly designed MAGEC X is currently the only device available in the United States.
This recall led to potential MAGEC system lawsuits for injuries, including skin discoloration, broken rods, implant failure and the need for painful additional surgeries.
Prior to this recall, NuVasive had issued a recall in June 2019 because devices had a higher-than-expected locking pin failure rate of 5%.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.