Reverse Shoulder Replacement Risks, Complications and Device Concerns
Reverse shoulder replacement is a common procedure for patients with rotator cuff damage, but some implants have been recalled or linked to serious complications that required additional surgery. Some people filed lawsuits after their devices failed and injured them.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Last update: November 17, 2025
- Est. Read Time: 5 min read
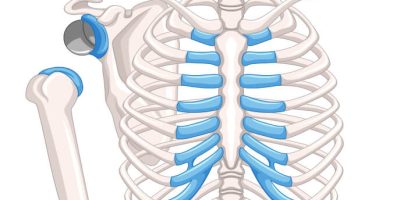
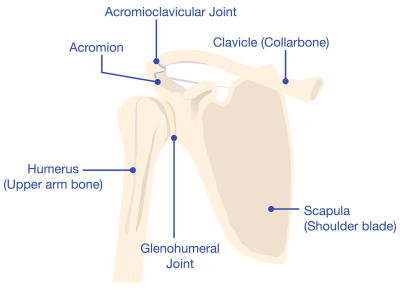
A reverse shoulder replacement is a surgical procedure that uses artificial implants, such as a ball and socket, to mimic the shoulder joint.
A standard shoulder replacement attaches an artificial socket to the shoulder blade and an artificial ball to the top part of the arm bone, which more closely mimics the natural shoulder joint. In a reverse shoulder replacement, the ball attaches to the shoulder blade, and the socket is at the top of the arm bone.
- Reverse shoulder replacements help alleviate chronic pain in people with rotator cuff tears.
- Manufacturers have recalled some devices, which may fail early.
- Early device failure led people to file lawsuits.
Doctors use a reverse shoulder replacement for specific injuries, such as a torn or malfunctioning rotator cuff. People may also be candidates for reverse shoulder replacement if they have had a previous shoulder replacement that wasn’t successful.
While this surgery can help people with chronic shoulder pain, reports of poor design and early failure led some people to file reverse shoulder replacement lawsuits against implant makers. The U.S. Food and Drug Administration (FDA) also informed the public about recalls for early device failure and the potential for additional surgeries.
Common Complications After Surgery
Potential risks and complications following a reverse shoulder replacement surgery include infections and blood loss, similar to any joint replacement. However, reverse shoulder replacements also have additional, unique complications. For example, dislocation is a common complication with reverse shoulder replacement.
- Anesthesia complications
- Blood vessel injuries
- Dislocations or joint instability
- Fractures of the shoulder bone
- Infections
- Nerve damage
- Reduced range of motion and decreased shoulder strength
According to a study from the journal Clinics in Shoulder and Elbow, complications occur in 15% to 24% of reverse shoulder replacements.
Some pain or numbness after the procedure is normal, but patients who experience swelling, bleeding, fever or chills should speak to their doctor right away.
Signs Your Reverse Shoulder Implant May Have Failed
Some post-surgery symptoms — such as persistent pain, swelling, difficulty using your arm or muscle weakness — could mean the implant has failed.
In addition to these physical symptoms, there may be grinding or other noises from the implant.
Implant Risks
Despite potential side effects, a surgeon may still recommend reverse shoulder replacement if it may improve a patient’s quality of life. Patients should be aware of the potential complications from this surgery and how they may affect their health.
Dislocation
A 2017 study of nearly 1,500 reverse shoulder replacements found that 69% of dislocations happened in the first three months after surgery. Dislocations were also more frequent among people who’d had surgery to repair or replace an earlier implant.
A reverse shoulder implant can dislocate if a patient lifts something or puts other tension on the muscles. Dislocation can also happen if the parts of the implant don’t match in size.
Doctors may be able to relocate the ball into the socket of the implant and put the patient’s arm in a sling for a few weeks. If this is ineffective or infeasible, the patient may require surgery to relocate the implant.
Infection Risk After Surgery
Infection is a risk of any surgery, especially implant surgeries. Some estimates suggest that one in 10 people who receive a reverse shoulder implant may get an infection. 
Germs that enter the body through a break in the skin can cause surgical site infections. In most cases, doctors can treat them with antibiotics.
If the drugs do not work, the patient might require another surgery to stop the infection. This revision procedure may involve cleaning the prosthetic and infected tissue or, in more severe cases, removing and replacing parts of the implant.
Loosening and Fractures
A patient’s bone health can play a part in fractures or the implant coming loose. Fractures can occur even as a surgeon places an implant into a patient for the first time. Patients receiving this joint replacement may already have weak bones that are more prone to breaking.
If the patient’s bone fails to grow around the implant’s baseplate, it may remain loose and require further surgery to correct.
Nerve Damage
Reverse shoulder implants can cause a loss of feeling or control in the arm and hand.
The implant can affect a bundle of nerves called the brachial plexus. These nerves run down the neck, through the shoulder and continue down to the hand.
The implants can put pressure on this nerve bundle or push it aside from its normal location. If this happens, a patient can lose feeling or even lose partial function of the arm and hand. Scar tissue from the surgery can cause similar nerve complications.
FDA Recalls and Safety Concerns About Reverse Shoulder Devices
The FDA issued its most serious Class I recall for the Zimmer Biomet Comprehensive Reverse Shoulder System Humeral Tray in 2017. The FDA issues Class I recalls for products that may cause serious injury or death.
Zimmer Biomet recalled the device because of design defects leading to a higher-than-normal rate of fractures and the potential for revision surgery.
In January 2024, the FDA issued a Safety Communication for the Exactech Equinoxe Shoulder System for defective packaging. The defective bags were missing barrier layers that protect the device from exposure to oxygen, potentially causing the device to wear down and fail early.
What To Do if Your Reverse Shoulder Implant Failed
If you suspect your reverse shoulder system may have failed, talk to your doctor right away. They will evaluate your shoulder implant using physical examinations, X-rays and other diagnostic exams as needed to confirm device failure.
If your device has failed, your doctor will discuss your options, which may include physical therapy, medication or revision surgery to replace the failed implant.
What Is Revision Reverse Shoulder Replacement?
Revision reverse shoulder replacement is a complex procedure intended to replace a problematic reverse shoulder implant. It may not be as effective as the initial shoulder surgery, but revision can help improve pain and function after a device failure.
A literature review published in Orthopaedics & Traumatology: Surgery & Research suggested a success rate of about 90% in revision surgery. But the study authors also noted it may take several procedures to reach this success rate.
Some people who needed revision surgery after an implant failed have filed lawsuits against device manufacturers.
Steps To Take if You’re Considering Legal Action
If you are considering taking legal action against a reverse shoulder replacement device manufacturer, keep all surgical and medical records. This includes hospital notes and details about the implant model.
These medical records will be important evidence that an attorney can use to see if you qualify for a claim and prove your case in court. If you can’t find all of your records, an attorney may be able to help you locate them.
When you’re ready to hire an attorney, make sure they have experience filing complex medical device injury claims. Take action right away because you have a time limit (called the statute of limitations) to file your claim, and it varies by state.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.