Bair Hugger Warming Blankets
The Bair Hugger Normothermia System is a forced-air warming, or FAW, system designed to prevent hypothermia and keep patients at a normal core body temperature during all three phases of orthopedic surgery — preoperative, intraoperative and postoperative.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Dr. John A. Daller
- Last update: December 1, 2025
- Est. Read Time: 7 min read
Hospitals have used Bair Hugger devices on more than 200 million patients since 1987 and continue to do so today. The warming blankets stabilize core temperature ahead of surgery, then maintain it during the procedure and immediately afterward as the patient recovers.
The current Bair Hugger warming units include models 505, 750, and 775. Model 500 is no longer in production. 3M, the company that manufactures the devices, also makes disposable Bair Hugger blankets and gowns.
How Bair Hugger Therapy Works
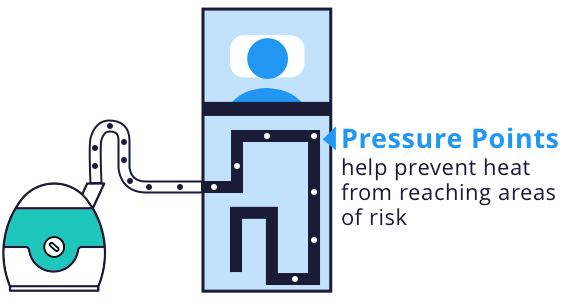
Bair Hugger warming units filter air and then force warm air through disposable blankets, which cover patients before, during and after surgery. These blankets are designed to use pressure points on the patient’s body to prevent heat from reaching areas at risk for pressure sores or burns. The blankets also include drain holes where fluid passes through the surface of the blanket to the linen underneath, which is supposed to reduce the risks of skin softening and unintended cooling because of heat loss from evaporation.
Bair Hugger blankets are disposable to reduce the chance of infection transmission from one patient to another. They are not designed to enter sterile fields during surgery.
3M recommends changing the HEPA filter in Bair Hugger units every 12 months or 500 hours of use, according to an April 2022 article in Infection Control & Hospital Epidemiology. Study authors found that many of these units reach 500 hours of use well before the 12-month mark.
The Bair Hugger may increase the risk of infection by drawing up air from contaminated floor, through a contaminated hose or a dirty filter, study authors said.
“The fact that Bair Hugger devices have been shown to potentially spread human pathogens across open surgical wounds should alert healthcare institutions to strictly follow the recommended filter replacement after 500 hours of run time rather than simply changing the filter on an annual basis.”
Bair Hugger History
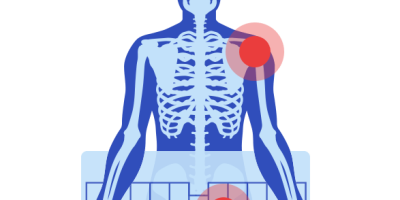
Dr. Scott D. Augustine invented the Bair Hugger in 1987 to keep patients warm during surgery. Almost all patients become hypothermic – a condition causing the body to lose heat faster than it can produce heat – during surgery. Even mild hypothermia during surgery can lead to blood loss, infections and prolonged hospital stays. The Bair Hugger equipment includes blankets, warming units and accessories.
Augustine began selling Bair Huggers through his company, Augustine Medical, 30 years ago. The company reorganized under new owners in 2003 and was renamed Arizant. In 2010, 3M paid about $810 million to buy Arizant, and it continues to market Bair Hugger products today.
“I am very proud of the old technology, but I am also proud to spread the word that there is a problem.”
That same year, Augustine’s company began selling a warming device called “HotDog,” which is a direct competitor to Bair Hugger. Augustine now warns against using Bair Huggers. He urges particular caution for joint replacement surgery. Augustine claimed the device posed an increased infection risk.
Hundreds of patients have filed lawsuits against 3M. The lawsuits claim Bair Hugger devices were responsible for infections they experienced following surgery.
Can Warming Blankets Increase Infection Risk?
Bair Hugger’s maker, 3M Company, claims that the devices are safe. It says surgeons have used them in millions of surgeries without problems. But Bair Hugger lawsuits claim the devices caused patients’ infections.
Studies have looked at how forced-air warming (FAW) devices affect air currents. Researchers looked at their potential to move small particles around operating rooms. Some studies found changes in air currents. Lawsuits claim this can carry contaminants into open incisions.
Bair Hugger Infection Risk Studies
-
2011: Deep-Joint Infection
A 2011 study published in The Journal of Bone and Joint Surgery simulated hip replacement and lumbar spinal procedures using both forced-air warming and conductive fabric warming, with a mannequin as a patient. In 2011, researchers compared forced-air warming, used in the Bair Hugger device, to an alternative-warming device that did not use forced air. They simulated a hip-replacement surgery and found that the forced-air device generated air currents that “mobilized floor air into the surgical site area.”
“Air-free warming is, therefore, recommended over forced-air warming for orthopedic procedures,” the researchers concluded. -
2011: Convection
Other researchers in 2011 conducted a similar study that was also reported in The Journal of Bone and Joint Surgery. They released neutrally buoyant detergent bubbles in a simulated surgery and tracked whether the bubbles moved to the simulated incision. When forced-air warming was used, they found “a significant mean increase” in the number of particles over the surgical site. They found forced-air warming sent more than 275 times as many bubbles over the simulated incision.
-
2012: Increased Air Temperature
A 2012 study in the UK journal Anaesthesia found similar results, but cautioned that the simulations were not perfect. Researchers pointed out that it was difficult to recreate the airflow generated or affected by multiple health care professionals working in an operating room. They also said that airflow should be one of several factors taken into account in determining what type of heating device to choose for a procedure. Waste heat created convection currents that created turbulence over the patient, which could draw damaging particles from below the operating table to the surgical site.
-
2013: Visualizing Forced-Air Warming
A 2013 study published in The Bone & Joint Journal found that waste heat from forced-air warming blankets such as the Bair Hugger, “can increase the temperature and concentration of airborne particles” around patients in operating rooms, increasing the chance of infection. Researchers in the study also simulated knee replacement surgeries. They compared simulations using a forced-air warming blanket to those using a warming device that did not use forced-air. They found that air "turbulence over the patient" contained 2 million airborne particles per cubic meter in the forced-air simulation. That is compared to just 1,000 particles from alternative warming methods. Researchers said their study did not show an increased infection risk. But they said certain types of operating room set-ups “can significantly disrupt” airflow. They warned that this could “draw particles from the potentially contaminated area below the sterile surgical field.”
Critics say that these studies have flaws that make them inconclusive or unreliable. 3M says that no study has ever established any link between Bair Huggers and infection risk.
Bair Hugger's FDA History
The U.S. Food and Drug Administration (FDA) cleared the first Bair Hugger warming system in 1987 through the 510(k) premarket clearance program as a substantially equivalent device. Under the 510(k) program, the FDA can clear a medical device without clinical proof that it is safe if it is similar enough to a previous device already on the market.
The FDA issued 12 additional clearances for Augustine Medical devices through the 510(k) premarket program from 1990 through 2002. After Arizant took over the Bair Hugger product line, the FDA issued two more clearances for medical devices through the 510(k) program from 2004 through 2006.
Over the course of its 30 years on the market, numerous adverse events have been reported to the FDA. The majority of early adverse event reports involved burns from prolonged exposure to heat during surgery or equipment malfunctions that resulted in cold air circulating under the blanket.
FDA ‘Actively Monitoring’ Bair Hugger Concerns
In August 2017, the FDA sent a letter to health care providers about FAW devices. The Administration said it was aware that people may avoid the devices due to concerns over possible infection risks. The letter pointed out that warming devices had a successful record and credited the devices for less bleeding, faster recoveries and decreased infection risk.
The agency said it “continued to recommend” use of FAW devices such as Bair Hugger. The letter said the FDA analyzed data, research and medical guidelines before coming to its decision.
“The FDA will continue to actively monitor this situation and will update this communication if significant new information becomes available,” the letter concluded.
Bair Hugger Recall
In January 2018, Bair Hugger recalled 165,000 warming blankets due to a design defect. The recall was not related to infection risks. The company said a design change could prevent some blankets from fully inflating. This could lead to dangerous changes in body temperature during surgery.
“If blankets are only partially inflated during use in surgery, the potential exists for incomplete warming therapy to be given to a patient,” 3M said in a Field Safety Notice.
The FDA received a report in January 2018 of a blanket that failed to fully inflate. A patient suffered hypothermia as a result.
The patient’s body temperature dropped to 95 degrees during the surgery. Doctors moved the patient to the intensive care unit. The patient remained there until regaining normal body temperature.
3M later recalled the lot with the blanket used in the surgery.
The worldwide recall affected 33,108 cases of Bair Hugger blankets. Each case contains five blankets.
Distributors shipped the affected lots to hospitals around the U.S. and 17 other countries. The affected lots were all distributed after October 26, 2017.
- R10359
- R10360
- R10361
- R10362
- R10363
- R10364
- R10365
- R10366
Thousands File Suit Over Bair Hugger Infections
People have filed more than 4,000 lawsuits over Bair Hugger devices.
Bair Hugger lawsuits claim the device led to infections that required as many as 27 additional surgeries and even leg amputations.

A judicial panel combined the lawsuits in a multidistrict litigation (MDL) in a Minnesota federal court.
MDLs allow several similar cases to be combined in a single action to save time and money as they move through the legal system.
3M won the first bellwether trial in May 2018. The court ordered a second bellwether ready for trial in December 2018.
The MDL judge told both sides to choose as many as nine bellwether cases.
The outcome of these trials may determine if the other cases go forward and could factor into the amount of any settlement.
Bair Hugger and Hip or Knee Replacement Surgery
Many of the people filing Bair Hugger lawsuits underwent joint replacement surgery.
Joint replacement involves unique infection risks. If a single bacterium lands on the implant during surgery, it can grow into a serious infection.
Bacterial joint infection can cause septic arthritis. This can cause sudden pain and swelling. It can destroy a joint if not treated with antibiotics right away. In some cases, a patient may need surgery to remove the implant and the infection. In rare cases, the infection can result in amputation.
Many of the people filing Bair Hugger lawsuits suffered infection after joint replacements.
3M Defends Bair Huggers
In response to Augustine’s claims, these studies and increasing numbers of lawsuits, 3M launched a campaign to prove Bair Huggers were safe. 3M said Augustine’s claims are alarmist and based on faulty studies and offers as evidence the results of more than 170 studies and more than 60 randomized clinical trials that show the system’s benefits, efficacy and safety.
In 2016, 3M published a pamphlet entitled “Let’s spread good science,” in which it maintained that an independent review of literature about FAW systems by ECRI Institute, an independent organization, found “insufficient evidence to establish that the use of FAW systems leads to an increase in SSIs (surgical site infections) compared to other warming methods,” and claimed that studies to the contrary used flawed methodologies.
“There is no evidence that forced-air warming increases the risk of infection,” 3M attorney Christiana Jacxsens told Outpatient Surgery Magazine in 2015. “In its entire history, not one hospital, doctor or medical provider has reported a single confirmed incidence of infection believed to be caused by the Bair Hugger device.”
Dr. Javad Parvizi, Professor of Orthopedic Surgery, Rothman Institute at Thomas Jefferson University, analyzed the evidence presented that FAW increased infections. He said, “There is no scientific proof that the use of forced-air warming blankets leads to an increase in surgical site infection regardless of the type of surgical procedure and the type of operating room.”
Alternative Treatment Options
For patients who want to avoid operating theatres where forced-air warming is used, there are alternatives. One alternative uses blankets with conductive fabric made with at least one layer of resistive polymers. Another way of keeping patients warm uses a mattress or garments with circulating warm water. A third alternative is a mattress with a conductive heating surface.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.