Bard PowerPort
A Bard PowerPort is a device doctors use to administer treatments like chemotherapy or fluids through a vein. However, many people say their PowerPorts broke or failed, causing serious injuries. These problems have led to lawsuits against the device’s manufacturer.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
This site is an amazing resource! I found the detailed information about the pro...
- Legally reviewed by Brendan A. Smith, Esquire
- Last update: January 30, 2026
- Est. Read Time: 5 min read
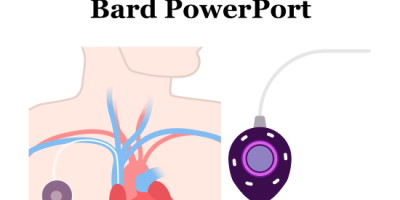
What Is a PowerPort and Why Is It Used?
A Bard PowerPort is a small medical device placed under the skin that helps doctors access veins more easily. It’s often used for patients who require frequent treatments, including:
- Blood transfusions
- Chemotherapy
- IV fluids
- Nutrition support
Doctors can also use it to take blood samples.
The PowerPort connects to a thin tube called a catheter, which goes into a vein. When used with a special needle, it can handle strong injections, including contrast dye used in medical scans.
Since the port is placed under your skin, it can remain in place for an extended period, making it easier and safer if you require repeated treatments or tests.
PowerPorts were manufactured by Bard, formerly known as C.R. Bard, a medical device manufacturer that is part of Becton, Dickinson and Company.
Unfortunately, PowerPort failures have led to hundreds of lawsuits claiming design defects led to complications and severe injuries.
Reported PowerPort Failures and Complications
Lawsuits and reports of adverse events to the U.S. Food and Drug Administration (FDA) have raised concerns about Chronoflex AL used in Bard’s catheters. The material is made from barium sulfate and polyurethane.
Litigation claims that the barium sulfate in the Chronoflex AL may weaken or cause the catheter to break down over time.
- Catheter Breakage or Fracture
- As time progresses, the catheter can weaken, causing pieces to break off and travel in your bloodstream. This can lead to serious health risks, including heart issues.
- Device Migration
- A deteriorating catheter might move from its original position, causing discomfort and possibly requiring surgery to fix.
- Port Malfunction
- Aging catheters can develop pits that allow bacteria to collect, increasing your risk of infection and adversely impacting treatments.
Failures can lead to various injuries, some of them severe or life-threatening. Of the 500 adverse event reports in Spring 2025, there were 155 reported cases of blood clots and 12 reports of pulmonary embolisms.
- Blood Clots
- Blood clots can form around the device, causing issues like deep vein thrombosis, which can potentially move to your lungs, resulting in a pulmonary embolism.
- Cardiac Tamponade
- This is when fluid collects around your heart, causing pressure that keeps your heart from pumping blood normally and potentially leading to shock, organ failure or death if not treated.
- Infections
- Some patients have had infections at the site of the medical device or in their blood, which may require removing the device and using strong antibiotics.
- Organ Puncture
- A broken catheter can cause fragments to move through your bloodstream, possibly damaging vital organs and causing internal bleeding.
- Severe Pain
- If a catheter detaches, it can cause intense pain that may need medical attention to manage.
- Vascular Damage
- Damage to your blood vessels can adversely impact blood flow and lead to complications like tissue death and clots.
Signs of Serious PowerPort Complications
If you have a Bard PowerPort, it’s important to be aware of the symptoms of a pulmonary embolism. This is a serious condition that can affect your lungs, and the symptoms can vary.
Pulmonary embolisms are life-threatening and require immediate medical attention.
- Chest pain
- Clammy or discolored skin
- Coughing up blood or blood-streaked mucus
- Dizziness or lightheadedness
- Excessive sweating
- Fainting
- Fast or irregular heartbeat
- Fever
- Leg pain or swelling, especially in the lower back area of your leg
- Shortness of breath
If you or someone you know has these symptoms, seek medical attention immediately. Have someone drive you to an emergency room or call 911.
Why Are PowerPorts the Subject of Lawsuits?
People have filed lawsuits against the Bard PowerPort, claiming that the device can malfunction and cause severe health problems.
PowerPort lawsuits allege that Bard used excessive amounts of barium sulfate in the catheter material. Using too much of this compound can weaken the device’s catheter and result in serious injuries.
“Many of our clients suffered serious complications after their Bard PowerPort device fractured or migrated,” attorney Moshe Horn of Simmons Hanly Conroy told Drugwatch. “We’re seeing cases involving bloodstream infections, blood clots, organ punctures, and in some instances, permanent nerve or cardiac damage. These aren’t minor issues. They can require surgery, long-term care, and in the worst cases, they’ve been life-threatening.”
People who were hurt say the device was unsafe and want the manufacturer to be held responsible. Lawsuits claim Bard failed to test and monitor the device adequately. Plaintiffs also say that Bard did not properly report PowerPort complications.
In August 2023, a federal panel combined 62 Bard PowerPort lawsuits into a multidistrict litigation (MDL) in an Arizona federal court. MDLs enable the consolidation of multiple similar lawsuits in a single litigation, making the legal process more efficient.
As of February 2026, there were 2,674 lawsuits pending in the Bard MDL.
Who Is Eligible To File a Bard PowerPort Lawsuit?
You might be eligible to file a Bard PowerPort lawsuit if you had one of the devices implanted on or after January 1, 2000, and suffered specific complications or injuries related to the defects named in other PowerPort lawsuits.
- Device fracture
- Erosion of the port’s reservoir through your skin
- Infection
- Thrombosis (blood clots)
You must have had the catheter port removed, attempted to have it removed, had it replaced or experienced a situation where your doctor was unable to replace it.
How To File a Bard PowerPort Lawsuit
If you are considering a PowerPort lawsuit, you can get a free case evaluation from Drugwatch. Simply complete a short form, and a member of our team will contact you to discuss your situation and determine if you have a valid case.
Our evaluations are completely free and come with no obligation to proceed. Regardless of your decision, you will receive valuable information and support.
Drugwatch partners with trusted law firms that specialize in personal injury and medical device litigation. Our goal is to connect you with an attorney who understands your unique situation.
FDA Oversight, Safety Alerts and Manufacturer Action
The FDA has received hundreds of adverse event reports related to the Bard PowerPort. The FDA’s MAUDE database includes 500 reports between April 21, 2025, and June 30, 2025 alone.
Despite this, there have been no Bard PowerPort or FDA recalls due to the device deteriorating over time. Bard mentioned this in a 2023 statement after the MDL was announced.
“Importantly, there has been no recall of any Bard PowerPort device due to catheter fracture,” the statement read. “We have identified no new safety risks related to our Bard PowerPort devices that are not already identified and communicated in each device’s instructions for use.”
However, Bard recalled 178 PowerPort devices in October 2019 due to another issue. Some of the devices may have been shipped with a connection designed for a different type of catheter, which could prolong the surgery required for implantation.
There have been several different Bard PowerPort models over the years. Since the introduction of the Chronoflex material in 2015, all the devices have been approved for use through the FDA’s 510(k) process.
The FDA’s 510(k) process allows a new device to be cleared if it’s shown to be as safe and effective as one already on the market. It doesn’t have to be identical, but it must function the same way without raising new safety concerns.
Approximately 99% of medical devices have been approved through the 510(k) pathway since the 1970s. Critics argue that this process may allow risky devices to reach the market because it lacks specificity and lets companies cite currently marketed devices in a potentially unsafe way.
-
November 20, 2015:
The FDA clears power-injectable implantable ports with Bard’s Chronoflex polyurethane catheters through the 510(k) process, noting it was substantially similar to previous devices on the market.
-
July 8, 2019:
FDA clearance is granted for updated PowerPort models using the 510(k) process, confirming continued substantial equivalence to previous PowerPort models.
-
December 8, 2023:
The FDA clears PowerPort ClearVUE Slim ECG Enabled Implantable Port and related models through the 510(k) process, indicating ongoing oversight.
-
October 31, 2024:
PowerPort ClearVUE Slim Implantable Ports receive 510(k) clearance, maintaining their Class II status.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.