NuVasive Precice System: Risks, Side Effects & Lawsuit Information
The NuVasive Precice system, a limb-lengthening implant, has been linked to complications like device breakage, metal toxicity and bone problems. FDA recalls and lawsuits allege the device puts patients at serious risk.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Last update: December 1, 2025
- Est. Read Time: 6 min read
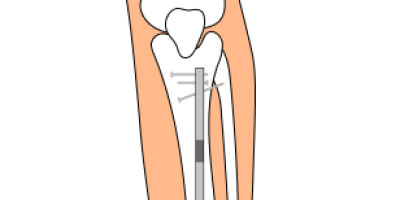
What Is the NuVasive Precice System?
The NuVasive Precice system is a surgically implanted limb-lengthening device used to treat limb length discrepancy (LLD) in your tibia, femur or humerus. LLD is a condition where one of your legs or arms is shorter than the other.
- Bone transport procedures (regrowing bones in areas where bone is missing)
- Mal-unions (fractures that don’t heal properly)
- Non-unions (fractures that don’t heal at all)
- Open and closed fracture fixation (procedures to fix broken bones)
- Pseudoarthrosis (when bones don’t fuse properly)
The NuVasive Precice system consists of an implantable metal rod (called a nail) with a magnetic motor that is placed inside your bone canal. After surgery, you adjust the implant with a hand-held external remote control that slowly lengthens your bone over time.
| Types of NuVasive Precice Systems | |
|---|---|
| Precice Bone Transport | Stainless steel |
| Precice Freedom | Titanium |
| Precice Intramedullary Limb Lengthening (IMLL) | Titanium |
| Precice Max | Titanium |
| Precice Nail | Titanium |
| Precice Plate | Stainless steel |
| Precice Short | Titanium |
| Precice Stryde | Stainless steel |
| Precice Unyte | Titanium |
The system is an alternative to traditional external fixator limb lengthening devices, but it has raised safety concerns due to reports of pain and bone abnormalities.
Complications and Side Effects
People who received NuVasive Precice implants have reported a wide range of side effects, including device breakage, pain, infections, revision surgery and more.
Device Fractures and Breakage
NuVasive lists implant fracture and loosening as complications associated with the Precice system. These issues may necessitate revision surgery or other treatments.
The company has also acknowledged risks of device migration and corrosion in its product safety information. A 2021 study published in Acta Orthopaedica confirmed the potential for corrosion in Stryde implants.
- Bolts backing out of place
- Inadequate screw fixation
- Over-lengthening of your limb
- Problems with screw removal
- Uncontrolled limb lengthening
Case Study: Complications with NuVasive Precice Stryde Device
To correct her leg length discrepancy, Alyssa Osos of Michigan underwent surgery to receive the NuVasive Precice Stryde limb lengthening device. The device was made from BioDur 108 stainless steel.
After the implant, Osos had severe side effects, including heavy menstrual bleeding and pain in her reproductive organs. An ultrasound revealed a large ovarian cyst. Her condition worsened when she also developed chromium toxicity and toxic chemicals in her liver.
When the device failed, Osos used crutches to prevent stress fractures. She lost months of physical therapy progress and experienced significant discomfort.
Osos underwent revision surgery to remove the failed device, but x-rays showed leftover fragments around the implantation site. Follow-up appointments confirmed bone damage, chromium toxicity and ongoing health complications.
Bone and Tissue Damage
The U.S. Food & Drug Administration (FDA) has reported bone abnormalities, tissue problems and pain connected to the stainless steel Precice devices.
NuVasive also warns of the same issues as the FDA.
- Bone abnormalities
- Bone density decreases
- Bones healing incorrectly (delayed healing, non-union or mal-union)
- Corrective surgery to fix soft tissue problems
- Hip or knee stiffness
- Tissue contractions and loss of movement in your joints
- Tissue or bone pain
Metal Ion Concerns
NuVasive warns about potential biocompatibility issues from metal particles or ions that may wear off your implant and impact your body.
Biocompatibility measures how well a device and the material it is made of function in your body. When a device has biocompatibility issues, it may cause tissue reactions, inflammation, immune responses, cancer and other adverse reactions.
The stainless steel in Precice Stryde can include metals like nickel, iron, carbon and chromium.
- Bone loss (osteolysis)
- Inflammation or pain near the implant
- Neurological symptoms if ions spread in the body
- Possible risks to fertility or child development
- Skin or tissue discoloration (metallosis)
Pain, Infections and Revision Surgeries
For some patients, the side effects of Precice have been serious enough to require revision surgery. Other complications have led to lifelong health problems.
- Infections
- Nerve or blood vessel damage
- Pain
- Paralysis or loss of sensory or motor function
- Permanent deformity
Investigations into other potential long-term health problems of Precice devices, including cancer, chronic toxicity, developmental toxicity and reproductive toxicity, are underway.
They reviewed 41 studies involving 782 patients who used these nails to lengthen 983 leg segments. Some complications were minor, while others were severe and included changes in the treatment plan, failure to reach the lengthening goal or permanent complications.
Surgical and Recovery Complications
Like most surgeries, there are risks for allergic reactions to anesthesia, blood clots and pain during the recovery period. With NuVasive, a second removal surgery is necessary, increasing your risk of complications.
Recovery from bone lengthening surgery can take time, requiring ongoing physical therapy and regular follow-ups. The consolidation phase, where new bone hardens, can take several months.
If you are considering bone lengthening surgery, discuss these risks with your health care provider. Regular monitoring and adherence to post-surgery care are crucial to minimize complications.
NuVasive and FDA Actions, Recalls and Safety Warnings
Over the past five years, there have been multiple actions concerning Precice systems from NuVasive and the FDA. These have included recalls, market approvals and letters sent to health care professionals.
-
February 1, 2021
NuVasive issued a safety notice to alert health care providers not to use Precice medical devices in patients under the age of 18. NuVasive told doctors that the devices did not meet safety standards for long-term toxicity risks.
-
February 20, 2021
NuVasive issued a recall notification for Precice Stryde, Precice Plate and Precice Bone Transport devices. This recall was due to reports of pain and unusual bone issues where the different parts of the device fit together. NuVasive recalled the devices “out of an abundance of caution” and to complete biological safety testing.
-
April 1, 2021
NuVasive placed a shipping hold on Precice device systems due to continuing testing for biocompatibility.
-
July 8, 2021
The FDA issued a letter to health care providers after receiving reports of pain and changes in the nearby bone and soft tissue of Precice Stryde device patients. Doctors were advised to monitor for changes in the surrounding bone and soft tissue during regular radiographic monitoring.
-
December 1, 2021
One day after NuVasive issued a field safety notice, the FDA reported that the shipping hold on titanium Precice implants had been lifted. The FDA also revealed that testing suggested the benefits of titanium-based Precice devices outweighed potential risks.
-
March 15, 2023
The FDA announced that NuVasive received 510(k) clearance for the “expanded use of the Precice IMLL system (including Precice Short) for limb lengthening of the shin bone (tibia) and thigh bone (femur) in patients greater than 12 years old.”
-
June 28, 2023
The FDA reminded doctors they could use the Precice IMLL system for limb lengthening in the tibia and femur in patients over the age of 12. Additional usages were available for adults. A reminder to remove the device after one year was also included.
-
September 30, 2024
NuVasive recalled the Precice Max device because of what the FDA described as “design, manufacturing and documentation issues.”
The FDA urges health care providers to report any adverse events related to Precice devices to the MedWatch program.
Legal Issues and Lawsuits Against NuVasive
Complications with the NuVasive Precice system have led to lawsuits. Allegations focus on biocompatibility problems, especially with the stainless steel models.
Patients and their families claim NuVasive failed to warn patients and doctors about the risks associated with implanting the stainless steel devices. Plaintiffs also say NuVasive sold implants with defective designs and did not perform enough testing before marketing the products.
NuVasive has also been sued over its NuVasive MAGEC system spinal rods, another implant that uses a magnet-driven lengthening system. Patients complained about biocompatibility issues and that the endcaps on the spinal system rods could detach, causing tissue damage.
If you suffered complications like bone or tissue issues after receiving a NuVasive Precice implant, you may be eligible to file a lawsuit. You may also qualify if you required surgery for early removal of the implant or underwent revision surgery because of complications.
Attorneys rely on medical records and surgical history to determine if you are eligible to file a case. Be sure to compile documentation like your implant identification details, including the model and whether it was a stainless steel or titanium device, surgical reports, follow-up imaging and doctor’s notes that verify your injury.
Alternatives and Patient Safety Considerations
Limb lengthening is a surgical procedure that can be performed in different ways. The two main approaches are external fixators and internal devices like the NuVasive Precice system.
External fixators are the more traditional option. Surgeons attach the device to the outside of your leg with pins placed into your bone. You then adjust the fixator each day slowly to lengthen the bone.
Even though the device is bulky and visible, it’s a well-established technique that has been used for decades. Pairing this method with physical therapy can help keep your joints, muscles and nerves healthy during recovery.
By comparison, the Precice system is less visible because the rod is implanted inside your bone and adjusted with a remote. The dangers of this implanted device include corrosion, bone and tissue damage, biocompatibility issues and more.
Since both methods carry risks, it’s important to be well-informed of all options for limb-lengthening treatment. Ask your doctor about all available options, the risks and benefits of each and how complications will be managed.
Understanding the details of all options can help you make a safe and informed decision about the best procedure for you.
Next Steps for Patients Affected by Precice Devices
If you received a NuVasive Precice implant, review your medical records to confirm which device you received and whether it was part of a recall. This is important for medical and legal claims.
- Monitor your symptoms: Watch for pain, abnormal body changes or any signs of adverse effects.
- Talk to your doctor: Report any symptoms to your doctor immediately. It’s also wise to discuss your specific case with your provider, especially if you have biocompatibility concerns.
- Follow instructions for use: Adhere strictly to post-operative care guidelines. This includes limiting weight-bearing activities and scheduling the device’s removal after one year.
- Talk to an attorney: If you’ve been injured by a Precice device, you may be entitled to compensation. A personal injury attorney can determine if you qualify to file a lawsuit.
Staying informed about FDA safety warnings, recalls and ongoing litigation can help you understand your health risks and legal rights. To learn more about your legal options, visit Drugwatch’s NuVasive Precice lawsuit page.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.