Tasigna
Doctors use Tasigna to treat a type of blood cancer called Philadelphia chromosome-positive chronic myeloid leukemia. While it is often more effective than Novartis’ other drug Gleevec, Tasigna has more side effects, including heart rhythm disorders and sudden death.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Katherine V. Sarna, Pharm.D., BCPS
- Last update: November 10, 2025
- Est. Read Time: 6 min read
Tasigna (nilotinib) is an oral chemotherapy drug manufactured by Novartis. The U.S. Food and Drug Administration (FDA) approved it in 2007.
Doctors prescribe the drug to treat a specific type of blood cancer called Philadelphia chromosome-positive chronic myeloid leukemia. They may also recommend the medication to patients who did not respond well to other cancer treatments, such as Gleevec (imatinib).
The drug can cause several common side effects, such as nausea, itching, rash, fatigue and headaches. It may increase the risk of blocked arteries, potentially fatal heart rhythms called torsade de pointes and sudden death.
According to the FDA Adverse Events Reporting System (FAERS), the agency had received 24,432 reports of Tasigna side effects as of November 2025. Of those, the agency categorized 17,240 as serious cases, including 4,881 deaths.
People who took the drug and suffered heart or vascular problems, such as decreased blood flow to the legs, heart or brain, are filing Tasigna lawsuits.
Nilotinib’s Effects on the Body
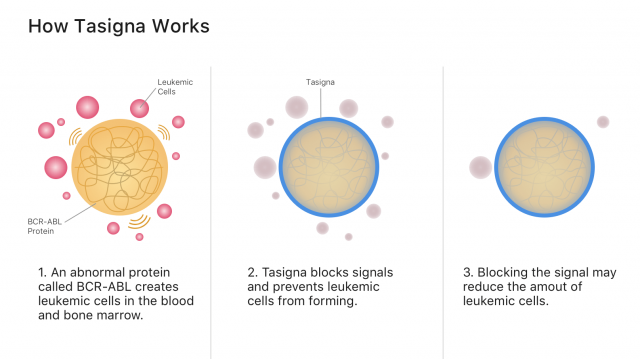
Tasigna’s active ingredient, nilotinib, works by blocking cancer-growing proteins called tyrosine kinases. It belongs to a class of drugs called tyrosine kinase inhibitors, or TKIs.
Specifically, nilotinib blocks signals from the abnormal blood proteins that create leukemia cancer cells. Blocking cancer cell signals may stop cancer from spreading and allow more healthy blood cells to grow.
If a patient achieves a major molecular response (MMR) after taking Tasigna for at least three years, their doctor may allow them to discontinue the drug. An MMR is when less than 0.1% of the cancer genes that cause chronic myeloid leukemia remain in the patient’s blood cells following treatment. MMRs are commonly considered to be major positive markers in a patient’s chronic myeloid leukemia journey.
Common Tasigna Side Effects
Common side effects of Tasigna can happen immediately after taking the drug. These are typically not serious and may go away after a short time. In clinical trials, the most common side effects occurred in 20% or more of participants.
- Anemia
- Constipation
- Cough
- Diarrhea
- Fatigue
- Headache
- Joint pain
- Nausea
- Night sweats
- Pain in the extremities
- Pruritus (itchiness)
- Raised body temperature
- Rash
- Stuffy or runny nose
- Thrombocytopenia (low platelet count that may lead to increased bleeding and bruising)
- Vomiting
Side Effects After Stopping Treatment
Patients who achieve good blood cell counts may be eligible to stop treatment. Doctors call this treatment-free remission, or TFR. However, stopping Tasigna may cause side effects.
People who try TFR may suffer more musculoskeletal problems after stopping the drug. These include pain in the muscles, arms, legs, joints, bones and spine. Patients who experience these symptoms should inform their doctor.
Serious Side Effects of Tasigna
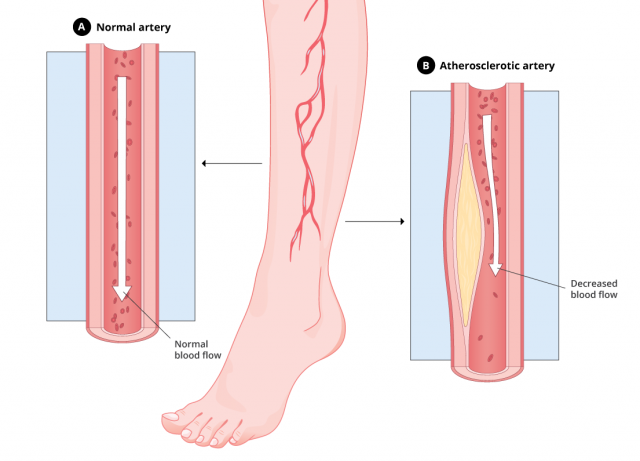
Experts have linked Tasigna to atherosclerosis, a vascular disorder in which the walls of the arteries thicken.
Atherosclerosis happens when fatty deposits build up in the blood vessels. This decreases blood flow to other parts of the body and may lead to a stroke or heart attack.
Treatment may include medications, surgeries or diet and lifestyle changes. The drug’s United States prescribing information does not warn specifically about this condition, but does warn about atherosclerosis-related conditions.
Health Canada warned the public in 2013 about atherosclerosis-related conditions associated with Tasigna. At the time, the agency said that its review of the Novartis global safety database revealed 277 atherosclerosis cases.
Peripheral Artery Disease: A Type of Atherosclerosis
A 2013 study in the journal Leukemia determined that PAD is “more frequently observed” in patients receiving Tasigna (nilotinib) than in those taking Gleevec (imatinib). Due to the potential severity of PAD, the study’s authors urged stringent monitoring and a “careful assessment of risk factors” regarding Tasigna.
Myocardial Ischemia
Myocardial ischemia is another possible side effect of Tasigna. It occurs when blood flow to the heart is reduced. The resulting lack of oxygen and blood starves the heart muscle, leading to myocardial infarction, or heart attack.
Atherosclerosis affecting the coronary arteries is the most common cause of myocardial ischemia. Treatment for myocardial ischemia may include diet and lifestyle changes, medication or surgery.
In a 2016 study published in the Annals of Internal Medicine, researchers studied 896 patients who took Tasigna or other similar drugs. They found that the rate for myocardial infarction (heart attack) was higher in people who took Tasigna compared to other similar drugs.
The most common symptom of myocardial ischemia is chest pain. It can feel like chest discomfort, heaviness, tightness or heartburn. Call 911 immediately if myocardial ischemia symptoms last for more than five minutes. These symptoms could mean a heart attack is occurring.
Antacids and Other Substances That May Interact With Tasigna
Several drugs may interact with Tasigna, including certain antacids, antibiotics, opioids and antidepressants. These drugs may increase or decrease in effectiveness when combined with nilotinib. They may also affect blood concentrations of nilotinib.
The most dangerous drug interactions can cause an abnormal heart rhythm due to an increased QT interval, the measurement on an electrocardiogram (ECG) that indicates how quickly the heart recharges after beating. These irregular heart rhythms may lead to sudden death. Grapefruit and certain herbal supplements (such as St. John’s wort) can also affect how Tasigna works in the body.
Potential Interactions With Tasigna
- Atazanavir: increased atazanavir exposure
- Clopidogrel: increased clopidogrel exposure
- Darunavir: increased nilotinib exposure
- Digoxin: increased QT interval
- Fluoroquinolones: increased QT interval
- Fluoxetine: increased QT interval
- Grapefruit juice: increased nilotinib exposure
- Haloperidol: increased QT interval
- Ketoconazole: increased QT interval
- Licorice: increased nilotinib exposure
- Losartan: increased losartan exposure
- Metoclopramide: increased QT interval
- Metoprolol: increased metoprolol exposure
- Pioglitazone: increased pioglitazone exposure
- Risperidone: increased QT interval
- St. John’s wort: decreased nilotinib exposure
- Venlafaxine: increased QT interval
- Warfarin: increased anticoagulation
This is not a complete list. Patients should inform their doctors of all medications and supplements they take.
Tasigna’s prescribing information lists several precautions and warnings. People with hypokalemia (low potassium), hypomagnesemia (low magnesium) or long QT syndrome (rapid, irregular heartbeat) should not take this drug.
Nilotinib may be harmful to a fetus. Women who may become pregnant should use an effective form of birth control while taking Tasigna. Women should not breastfeed during treatment or for at least 14 days after their final dose.
The drug’s capsules contain lactose, so patients who are lactose intolerant or have milk allergies should tell their doctors.
Tasigna vs. Gleevec
Like Tasigna, Gleevec treats Philadelphia chromosome-positive chronic myeloid leukemia, but it’s approved for other uses as well. Novartis manufactures both Tasigna and Gleevec, which the FDA approved in 2001.
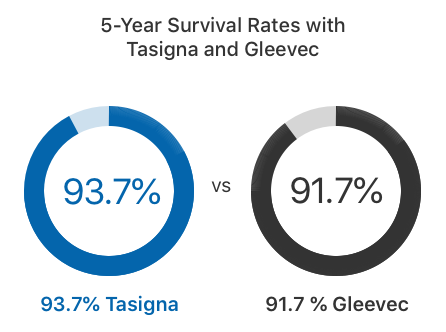
In clinical trials, twice as many adults who took Tasigna achieved an MMR within the first year of treatment compared to those who took Gleevec, according to the Tasigna label. About 93.7% of adults survived at least five years with Tasigna, while 91.7% survived with Gleevec.
In 2017, the FDA approved an addition to Tasigna’s label that says patients with early-phase chronic myeloid leukemia who have been taking the medication for at least three years may be eligible to stop taking it if their leukemia has sufficiently responded to treatment. Patients who take Gleevec don’t have the same option.
“Patients diagnosed with CML generally face a lifetime of treatment to keep their leukemia from growing or recurring,” Dr. Richard Pazdur, director of the FDA’s Oncology Center of Excellence, said in a press release. “Today’s approval shows that some patients may be able to stop treatment with Tasigna altogether if they are showing a strong response to therapy.”
But Tasigna may result in more serious side effects than Gleevec. For example, Gleevec’s labeling does not include a black box warning, the FDA’s most serious form of medication warning. Tasigna’s labeling features a black box warning for increased QT intervals and sudden death.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.