Tasigna Lawsuits
Health Canada issued a warning for the chemotherapy drug Tasigna after the agency found evidence that the medication could cause hardening and narrowing of the arteries, a condition known as atherosclerosis. Novartis failed to warn American cancer patients of the risk, according to lawsuits. Now, people are suing the company for injuries, such as stroke and death.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Julie Lawson Timmer, Esquire
- Last update: April 23, 2025
- Est. Read Time: 6 min read
Tasigna lawsuits allege the chemotherapy drug’s maker, Novartis, failed to warn Americans about the risk of atherosclerosis, a condition that causes arteries to harden, narrow or become blocked. Some people died because of related conditions, such as stroke.
In 2013, Health Canada alerted Canadians to the risk of atherosclerosis with Tasigna. Novartis added warnings to the Product Monograph used to inform Canadian doctors about the appropriate use of the drug, but the company did not add anything about atherosclerosis or related complications to its prescribing information in the United States.
A wrongful death case filed by Kristi Lauris ended in a confidential settlement in 2018. The case had been set for trial on Oct. 29, 2018. After the parties agreed to a settlement in August 2018, the judge cancelled the trial. The judge signed the Order Granting Petition for Approval of Settlement on Nov. 6, 2018.
Americans who suffered atherosclerosis or other cardiovascular problems after taking Tasigna may qualify to file a lawsuit for compensation.
In August 2021, over Novartis’s objection, the U.S. Judicial Panel on Multidistrict Litigation issued a Transfer Order centralizing all federal Tasigna lawsuits into a federal multidistrict litigation (MDL) before Judge Roy B. “Skip” Dalton, Jr., U.S. District Judge for the Middle District of Florida. The MDL was assigned MDL No. 3006 and includes federal court cases transferred from the Southern District of Illinois and the District of New Jersey.
As of April 1, 2025, 39 cases had been filed in the MDL, with 36 still pending. No orders regarding discovery or other pretrial matters appear to have been entered, according to our review of the court’s docket.
Tasigna suits have also been designated as multicounty litigation (MCL) in New Jersey state court. In April 2021, the Supreme Court of New Jersey designated all pending and future Tasigna lawsuits in the state as multicounty litigation (MCL), and assigned them to Superior Court Judge Rachelle L. Harz in the Bergen Vicinage (in New Jersey, geographical regions are called vicinages) for centralized management. Centralized management is not the same as consolidation, as made clear in Judge Harz’s Initial Case Management Oder on June 8, 2021.
Two years later, in April 2023, the cases were reassigned to Superior Court Judge Gregg A. Padovano, also in the Bergen Vicinage.
The MCL is ongoing. On August 12, 2021, the court appointed a Special Discovery Master to oversee discovery disputes between the parties. On February 25, 2025, Judge Padovano issued an order extending discovery in the MCL until December 31, 2025.
Atherosclerosis and Other Injuries
Tasigna has a few common and serious side effects, but the main side effect included in lawsuits is atherosclerosis. Normally, atherosclerosis occurs slowly over time because of factors such as poor diet or smoking. But people who took Tasigna may suffer “severe, accelerated and irreversible atherosclerosis-related conditions,” according to Lauris’ complaint.
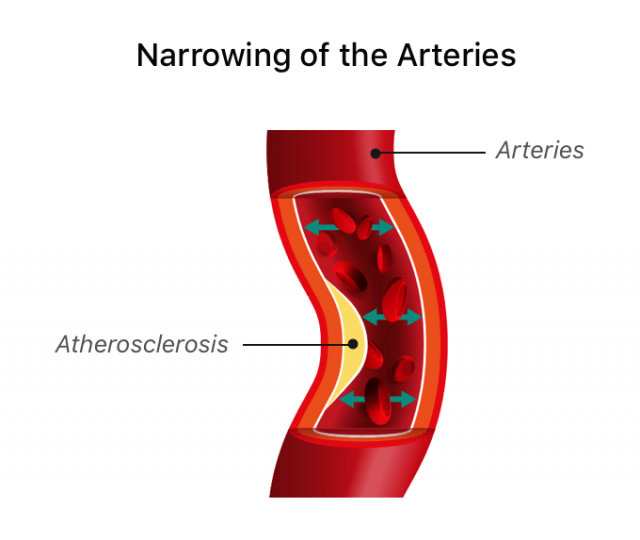
Because atherosclerosis affects many different blood vessels in the body, it can lead to several complications. These complications may also be included in lawsuits.
- Atherosclerosis
- Heart attack
- Stroke
- Blood clots
- Peripheral artery disease (narrowing of arteries in legs, stomach, arms and head)
- Myocardial ischemia (narrowing of arteries that feed the heart)
- Carotid artery stenosis (narrowing of arteries in the neck)
- Narrowing of arteries in the legs
- Narrowing of arteries in the brain
- Blockages in blood vessels
Accusations Against Novartis
Tasigna lawsuits accuse Novartis of several counts of wrongdoing. The main accusation is that the company failed to warn Americans of the risks of atherosclerosis.
“Novartis failed to warn of risks that Tasigna caused several forms of severe, accelerated and irreversible atherosclerosis-related conditions — i.e. the narrowing and hardening of arteries delivering blood to the arms, legs, heart and brain,” according to a lawsuit filed by Bruce Becker. “Since at least 2010, Novartis was aware that Tasigna caused severe, accelerated and irreversible atherosclerosis-related conditions.”
Becker took Tasigna and suffered a stroke caused by atherosclerosis.
According to Becker’s complaint, Novartis’ clinical investigators urged the company to warn patients and doctors about the risk, and it did not. It also had access to multiple medical studies linking Tasigna to accelerated atherosclerosis. The company’s own phase three randomized trials showed a higher risk when compared to the related drug Gleevec.
Other accusations against Novartis include:
- Aggressive and illegal marketing of Tasigna
- Intentionally failing to warn about Tasigna’s risks for the sake of profit
- Fraud and negligence
- Breach of its duty of care by not warning about risks
- Reckless conduct
- Conscious disregard or indifference to the life, safety or rights of people injured by Tasigna
People Who Filed Lawsuits
A few people have already filed Tasigna lawsuits over atherosclerosis and its complications. Becker and Lauris are among the plaintiffs and so are Dennis and Lori McWilliams.
Kristi Lauris
Lauris filed a wrongful death lawsuit against Novartis on March 22, 2016 after her husband, Dainis, died from atherosclerosis complications. Dainis showed no signs of atherosclerosis before he took Tasigna. In fact, before he took Tasigna, he had been taking Gleevec for about 10 years without any atherosclerosis issues.
He switched to Tasigna in October 2012. After he started taking the drug, he began suffering symptoms of atherosclerosis, including cramping and tightening in his legs and shins. At the time, neither he nor his doctor was aware that Tasigna could cause these problems because Novartis did not warn the public. So, he dismissed it as muscle cramps, but his condition continued to deteriorate until he could barely walk.
“Upon taking Tasigna, Dainis Lauris developed severe, accelerated and irreversible atherosclerosis-related conditions, which caused, among other things, 100-percent narrowing of his femoral arteries, 40- to 60-percent narrowing of his coronary arteries, and 70-percent narrowing of his cerebral arteries.”
About a year later, an angiogram found 100 percent blockage in his right femoral artery and 90 percent blockages in the arteries of the right and left knee. After his oncologist discovered an article connecting Tasigna to atherosclerosis in a medical journal, Dainis stopped taking Tasigna.
In an effort to avoid amputating his leg, he underwent more painful surgeries, but he died of complications from atherosclerosis in March 2014. An autopsy revealed narrowing of the arteries in his brain and heart.
“Upon taking Tasigna, Dainis Lauris developed severe, accelerated and irreversible atherosclerosis-related conditions, which caused, among other things, 100-percent narrowing of his femoral arteries, 40- to 60-percent narrowing of his coronary arteries, and 70-percent narrowing of his cerebral arteries,” according to Lauris’ complaint.
Bruce Becker
Becker filed a lawsuit against Novartis on Feb. 26, 2018 after taking Tasigna and suffering a stroke at 66 years old. Like Dainis Lauris, Becker had taken Gleevec prior to taking Tasigna.
When Becker started taking Tasigna, he had no atherosclerosis-related conditions. Shortly after taking the drug, he developed rapidly progressing atherosclerosis in his carotid arteries. This led to his stroke.
“[A]t no time before or during the time while Bruce Becker took Tasigna did the Tasigna label warn of the risks of atherosclerosis-related conditions associated with the drug,” his lawsuit states.
Dennis and Lori McWilliams
Dennis McWilliams’ doctor diagnosed him with Philadelphia chromosome-positive chronic myeloid leukemia in 2007 and initially prescribed him Gleevec. After running some tests his doctor switched him to Tasigna in June 2011. He suffered a stroke in August 2013.
McWilliams and his wife, Lori, filed a lawsuit against Novartis. In July 2018, Novartis filed a motion to have the case dismissed.
The company argued that there was no way the FDA would have approved a warning for atherosclerosis, and therefore, Novartis did not add a warning. Novartis also argued that McWilliams was not entitled to claim punitive damages and his wife’s claim for consortium should also be denied.
Judge Robin L. Rosenberg denied Novartis’ motion to toss out the case entirely but did agree that McWilliams could not claim punitive damages. Both parties agreed to a Voluntary Dismissal with Prejudice on Nov. 26, 2018.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.