Taxotere Lawsuits
Taxotere lawsuits allege permanent hair loss and eye damage, including vision loss, caused by the chemotherapy drug commonly used to treat breast cancer. As of February 2026, there were 1,335 lawsuits pending for hair loss and 374 pending for eye damage in Louisiana federal court. Litigation is ongoing and no settlements have been announced.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Trent B. Miracle, Esquire
- Last update: December 3, 2025
- Est. Read Time: 7 min read
Latest Taxotere Lawsuit Updates
As of February 2026, there were no Taxotere lawsuit settlement payouts to date in the hair loss or vision loss litigations. Hair loss lawsuits are consolidated in federal MDL 2740, while eye injury lawsuits are consolidated in federal MDL 3023. Both MDLs are before Judge Jane Triche Milazzo in Louisiana federal court.
Sanofi tried to dismiss all claims in the Taxotere eye injury MDL in 2022, but cases remain pending in 2024.
- December 2025: In a major win for people who have filed lawsuits, the judge overseeing the Taxotere lawsuits has denied Sanofi’s motion for summary judgment. This clears the way for the eye injury cases to continue advancing through the legal process and eventually work toward holding trials.
- October 2025: We are still waiting for a final decision from the judge on Sanofi’s summary judgment motion, which is critical to the future of these cases. It is not unusual for a decision to take some time. The hearing on that motion was held in August.
- August 2025: There have been no major updates among Taxotere eye injury lawsuits over the last few months. We are awaiting the outcome of Sanofi’s motion for summary judgment after a hearing was held on Aug. 19. The judge’s decision on that motion will have a huge impact on the litigation going forward.
- May 2025: The judge overseeing the Taxotere MDL focused on hair loss granted summary judgment to the defendants. This decision does not impact the eye injury litigation.
- February 2025: Sanofi has filed a motion for summary judgement in the Taxotere eye injury MDL, a move that could potentially end the litigation in its favor if approved. Sanofi has based its motion on claims that the plaintiffs do not have admissible expert testimony and that Taxotere’s label already warns of the eye injuries patients say they experienced. It is not overly unusual for defendants in MDLs to file for summary judgement, and it does not mean that the judge will approve it.
- October 2024: It has been a quiet few months without any major updates in the Taxotere eye injury MDL. We will continue to closely monitor the progress of the litigation.
- May 2024: Sanofi filed motions to dismiss several cases in MDL 2740, and plaintiffs have filed motions in opposition.
- March 2024: Some plaintiffs filed amended short form complaints and some cases and defendants have been dismissed. No trials have taken place in MDL 2740. Plaintiffs and defendants filed motions to dismiss, and discovery has not started in MDL 3023.
- November 2023: Taxotere litigation was still pending with 9,917 hair loss lawsuits and 249 lawsuits for vision problems in two separate MDLs in Louisiana federal court.
- April 2023: Taxotere litigation was still pending and there are 10,720 hair loss lawsuits and 168 lawsuits for vision problems in two separate MDLs in Louisiana federal court.
- March 2023: Judge Milazzo added an additional liaison counsel for another group of defendants, according to Pretrial Order No. 3, filed in February 2023.
- February 2023: Judge Milazzo asked defendants to provide a list of plaintiffs in the hair loss litigation who may be dismissed for not providing the required information in their court documents, specifically a Product ID and fact sheet deficiencies. That deadline was set for March 2023.
Sanofi won two bellwether trials in the hair loss litigation, one in September 2019 and another in November 2021. Litigation continues despite this outcome.
Why Are People Filing Taxotere Lawsuits?
People are currently filing Taxotere lawsuits because they suffered permanent eye damage, and studies have linked these injuries to Taxotere (docetaxel). Drug manufacturer Sanofi-Adventis failed to warn doctors and patients that Taxotere could cause permanent damage despite knowing about the risk for years, according to lawsuits.
Some patients who received Taxotere may suffer permanent damage to the lacrimal system in the eye. The lacrimal system is responsible for producing and properly draining tears that help keep eyes healthy. Lacrimal system damage can block tear ducts and cause excessive tearing which leads to vision problems.
Similarly, another group of lawsuits claims Sanofi failed to warn patients that hair loss from Taxotere may be permanent.
Taxotere Vision Damage Lawsuits
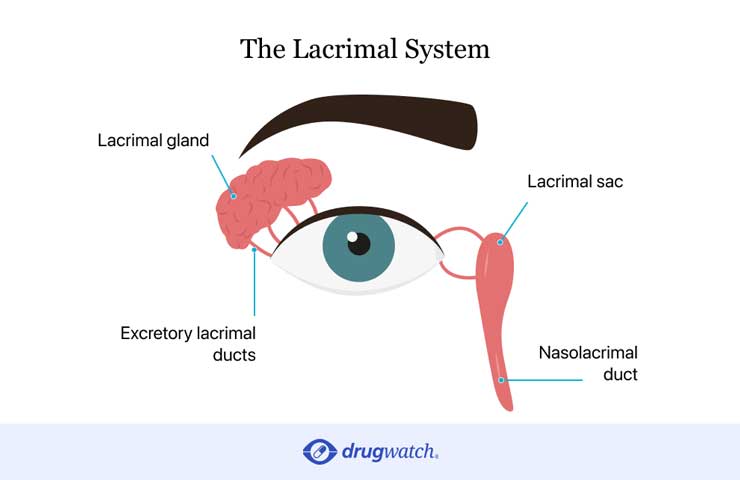
Studies show Taxotere may damage the lacrimal system and cause excessive tearing, a condition called epiphora.
Taxotere vision damage lawsuits filed against Sanofi-Aventis claim that the drug’s prescribing label doesn’t adequately warn patients and doctors about the potential for permanent eye injuries and other vision-related side effects of Taxotere. In addition, the label doesn’t tell patients with side effects to seek immediate treatment to avoid potentially permanent eye damage.
“Although Sanofi warns that ‘excessive tearing which may be attributable to lacrimal duct obstruction has been reported,’ Sanofi failed to warn patients and oncologists of the risk that the damage can occur quickly and can be permanent,” according to a Taxotere lawsuit filed by Jennifer Burns in 2021.
Plaintiffs had to undergo multiple surgeries to insert tubes into tear ducts to correct tearing problems. For some, surgeries didn’t alleviate epiphora.
Vision damage lawsuits were consolidated into multidistrict litigation in Louisiana under the same judge overseeing the hair loss lawsuits in February 2022.
Studies Show Permanent Eye Damage
Studies show Taxotere may damage the lacrimal system and can cause canalicular stenosis, or a blockage in the tear ducts. This leads to excessive tearing called epiphora. If canalicular stenosis isn’t treated quickly, it leads to permanent blockage of tear ducts.
Some patients who have taken Taxotere have suffered from permanent epiphora that causes blurry vision, irritation and swelling. The condition affects self-esteem, relationships and the ability to read or drive.
Researchers published the first studies linking Taxotere to epiphora as far back as the early 2000s. According to a 2001 study published in JAMA Ophthalmology, epiphora may occur in up to 77% of patients treated with weekly docetaxel treatments. Researchers hypothesize that docetaxel is secreted in tears and can cause fibrosis and scarring in tear ducts, which leads to canalicular stenosis.
Taxotere Hair Loss Lawsuits
Women and families suing allege the manufacturers were aware of the link between permanent hair loss and Taxotere (docetaxel) use and failed to warn physicians or patients. The lawsuits also allege the manufacturers marketed Taxotere (docetaxel) as more effective than other chemotherapy drugs even though other chemotherapy drugs were equally effective and did not include the risk of permanent hair loss.
Hair loss lawsuits were consolidated in Louisiana federal court in 2016.
Studies Showed Permanent Alopecia
According to lawsuits, Sanofi misled the public by falsely assuring them that hair would grow back after chemotherapy. But the company should have known that their drug had a higher rate of permanent alopecia than similar drugs on the market.
-
1998
Sanofi sponsored a study called GEICAM 9805. By 2005, the company knew that the results of this trial revealed 9.2% of women who used the chemo drug suffered permanent alopecia.
-
2006
Dr. Scot Sedlacek of the Rocky Mountain Cancer Centers conducted a study that revealed Taxotere could cause more than 6% of women to suffer permanent alopecia.
Despite informing patients in other countries, Sanofi did not warn women in the U.S. of this risk for years. The words “permanent hair loss” or “alopecia” did not appear in any information published in the U.S., lawsuits say.
Taxotere lawsuits call into question Sanofi’s motives since Taxotere’s initial FDA approval in 1996. They say Sanofi downplayed the risks of the drug and trained employees to misrepresent its safety and effectiveness.
According to a 2015 lawsuit filed by one of Sanofi’s former employees, the company engaged in illegal payment of kickbacks to health care professionals to prescribe the drug.
Do You Qualify for a Taxotere Lawsuit?
You may qualify for a Taxotere vision loss lawsuit if you received Taxotere chemotherapy drug for breast cancer or another type of cancer and suffered vision loss or problems. Each law firm has their own criteria, but in general the following injuries may qualify for a vision loss lawsuit.
- Blocked tear ducts
- Blurred vision
- Dry eyes
- Excessive watery eye or tearing (epiphora)
- Loss of vision
- Swelling in and around the eyes
- Narrowing of the tear ducts
- Eye irritation
- Eye surgery
Breast cancer survivors who were prescribed Taxotere before December 2015 and experienced permanent baldness, otherwise known as alopecia, as a result of Taxotere use may be eligible for compensation. Although hair loss is a common side effect related to chemotherapy drugs, permanent baldness is not.
Permanent baldness is a disfiguring condition, especially for women. Women who experience disfiguring alopecia as a result of Taxotere (docetaxel) use not only suffer physical and emotional loss, but also financial loss, including the cost of wigs and potentially the loss of work or inability to work due to significant psychological injury.
Not all law firms are still accepting hair loss cases. As of November 2022, no settlements or jury verdicts have been announced.
How to File a Taxotere Lawsuit
The first step in filing a Taxotere lawsuit is getting a free case review from a Taxotere attorney. Only a qualified attorney can tell you whether you have a claim. Lawyers in these types of cases do not charge a fee unless you receive a settlement or win your trial.
Before talking to an attorney, make sure you have medical records from your oncologist and eye doctor. If you have any receipts, notes or any other documents related to your eye problems and Taxotere use, have it available. The attorney will ask you some questions about your Taxotere experience.
If you decide to hire the law firm, the firm will send you some paperwork to sign. Hiring a lawyer is an important decision, make sure you are comfortable with the firm before signing any paperwork.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.