Knee Replacement Surgery
Knee replacement surgery, also called arthroplasty, replaces an injured or diseased joint with an implant to relieve pain and improve movement. Your doctor may recommend total knee replacement or partial knee replacement. Revision surgery is done to replace implants or address complications.
What Is Knee Replacement Surgery?
When the knee joint is damaged, people can experience pain and decreased range of motion. A doctor may recommend knee replacement surgery or arthroplasty, which involves replacing damaged weight-bearing parts of the knee joint with an artificial implant or prosthesis.
Since the first knee replacement surgery in 1968, advanced surgical techniques and implants have made it one of the most successful and common procedures. More than 600,000 knee replacements are performed each year in the U.S., according to the American Academy of Orthopaedic Surgeons.
The most common reason for knee replacement is arthritis that damages the cartilage and bones in the joint. Specific knee problems that might make surgery necessary include:
- Osteoarthritis: Age-related wear and tear on the knee joint and usually occurs in people older than 50.
- Rheumatoid arthritis: Causes the immune system to attack the membranes in the knee and creates damage and cartilage wear.
- Traumatic arthritis: Results from a serious knee injury (fracture, ligament damage, meniscus tear). This weakens the joint.
- Avascular necrosis: Affects teenagers and young adults. The bones in the knee become soft, causing the bone and cartilage to wear down. This usually resolves during adulthood, but in some cases the wear on the bone is extensive and arthritis sets in.
- Abnormal formation or alignment: Sometimes called being “knock-kneed” or “bow-legged,” it creates high stress on the joint and causes abnormal wear.
The majority of people experience an improvement in range of motion and quality of life after knee replacement surgery. Some people may experience complications such as infection or loosening of the device.
Types of Surgeries
Types of knee surgery include traditional total knee replacement, minimally invasive total knee replacement and partial knee replacement. Revision surgery may be necessary for those with older implants or when rare but when complications occur.
If knee cartilage and bones become damaged or worn, movement can be painful and difficult. Knee replacement surgery creates new surfaces on the bones using metal components and replaces cartilage with smooth plastic. Some doctors may refer to this as a knee “resurfacing” because only the surfaces of the bones are replaced.
Sometimes the bone is too damaged to spare so it’s cut and replaced. Extra bone may also need to be cut to fit specific implant models.
Knee replacement surgery can involve one or more damaged knee surfaces.
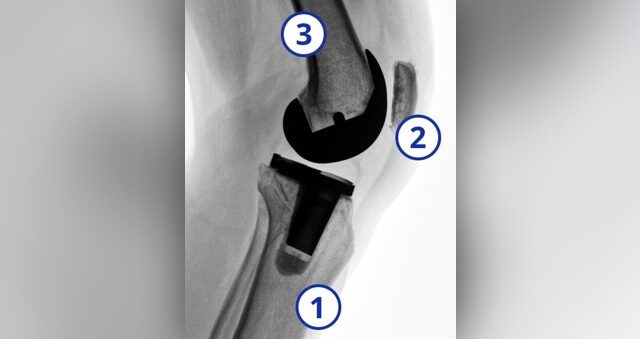
The three major knee surfaces are the:
-
1. FemurLower end of the thigh bone
-
2. PatellaKneecap
-
3. TibiaUpper end of the shin bone
Before deciding on surgery, your orthopedic surgeon will evaluate your medical history, overall health and your knee’s range of motion. An X-ray or MRI will show the condition of your ligaments, cartilage and bone. If you’re a candidate for surgery, your doctor will explain the different surgical procedures, their risks, benefits and potential disadvantages.
Knee replacement surgery usually takes about two hours, and general anesthesia is used. Your surgeon may use a robotic arm system – or computer-assisted surgery – to more accurately determine the damaged area and align the implants. A 3-D model of the patient’s joint allows them to align the implant more accurately.
Knee replacement devices (prostheses) include total implants and partial implants. Doctors recommend a partial or total knee replacement surgery depending on the number of surfaces that are damaged. A specific model of prosthetic may be recommended depending on the amount of damaged bone and cartilage that needs to be replaced.
Traditional Total Knee Replacement
During a total knee surgery, your surgeon makes an incision between 6 to 10 inches to expose the knee joint. The bottom of the femur is resurfaced and fitted with a metal piece (femoral head). Depending on the condition of the bone, your surgeon may have to cut more of the damaged bone off the femur.
Next, a small piece of bone is cut from the top of the tibia and a flat, weight-bearing metal piece is screwed into the top (tibial plate). Then, a flat piece of medical-grade plastic (menisci replacement plate) is placed between these two pieces to absorb shock and allow the knee to glide smoothly. The back side of the patella facing the end of the femur is also resurfaced and fitted with a small plastic button.
-
Step 1: Preparing the Bone
Damaged cartilage is removed at the ends of the femur and tibia, along with a small amount of bone.
-
Step 2: Positioning of the Implant
Damaged bone surfaces are replaced with metal components, and cartilage is replaced by medical-grade plastic.
-
Step 3: Resurfacing the Patella
Depending on the case, some surgeons may resurface the underside of the patella (kneecap) with a plastic disk.
-
Step 4: Inserting a Spacer
A piece of smooth, medical-grade plastic is placed in between the metal parts to facilitate ease of movement.
Doctors usually recommend total knee replacement surgery if all three of the joint’s compartments are damaged. This procedure replaces the end of the tibia, femur and the back side of the patella.
Minimally Invasive Total Knee Replacement
Some medical centers perform minimally invasive surgery for a total knee replacement, also called minimum incision joint replacement. Less muscle and tissue are cut, leading to less blood loss, increased range of motion and a shorter hospital stay. Many patients go home the same day and the recovery process is usually much quicker than traditional total knee surgery.
Generally, the best candidates for this are younger and in better health. Older patients and patients who have undergone knee surgeries in the past may not be candidates for minimal incision procedures.
Because it uses a much smaller incision, there’s a higher risk for poor implant placement compared to traditional total knee surgery. Other common complications include nerve and artery injuries, wound healing problems and infection. Only specialized medical centers typically offer this surgical option.
Partial Knee Replacement
With a partial knee replacement, your doctor only resurfaces one or two compartments of the knee. This alternative to a total knee replacement is for patients whose condition is limited to just one area of the knee.
The three major compartments of the knee include:
- Medial: Inside part of the knee
- Lateral: Outside part of the knee
- Patellofemoral: Front of the knee between the kneecap and the thigh bone
Surgeons spare as much of the healthy tissue and bone as possible. This may help regain range of motion, encourage more natural function and decrease healing time.
Other parts of the knee may become arthritic later and another surgery to resurface those portions of the knee might be required. Partial knee replacement can result in slightly less predictable pain relief compared to a total knee replacement.
Depending on the number of surfaces damaged, there are three general types of partial knee surgery:
- Unicompartmental surgery: Replaces one side (compartment) of the knee with metal and plastic. The healthy cartilage and bone, as well as all of the ligaments, are preserved.
- Bicompartmental surgery: Replaces both sides (medial and lateral) of the knee, while leaving the lateral bone areas and cartilage intact.
- Patellofemoral surgery: Replaces the end of the thigh bone with a metal piece and resurfaces the back of the patella (kneecap).
Each procedure requires a different prosthesis and surgical technique. The incision for a partial knee replacement is much smaller than for a total knee replacement — usually only about 3 to 4 inches.
After making the incision, your surgeon first makes sure that the damage can be corrected with a partial procedure. If that’s the case, the damaged parts of the bone are then capped with metal pieces, and a plastic piece is inserted between the two metal components.
Recovering from Surgery
After the procedure, you will be given antibiotics for about a day after surgery and anticoagulants to prevent blood clots. A drain to stop fluid from building up around the knee may be inserted.
To help retain flexibility and range of motion, some surgeons recommend a continuous passive motion machine that slowly bends and straightens the knee joint while keeping it supported. For most people, the knee sufficiently heals after long-term recovery and normal activities can resume.
Your return to work depends on your individual situation. Office workers, for example, who sit at a desk may return to work sooner than someone whose job is more physically demanding.
Managing knee pain following surgery is important for recovery. Your doctor will advise you on managing discomfort. Physical therapy and exercise to strengthen the joint are also important. Rehabilitation after surgery can take several months.
It’s normal to notice less flexibility because an implanted joint is not as flexible as a healthy knee joint. How flexible the joint was prior to your operation may influence your range of motion after surgery.
Complications That Can Arise
Complications are more likely to occur with total knee replacement than partial knee replacement. Some of these complications occur because of the surgery itself. Others occur because of a faulty implant and are the subject of ongoing litigation.
- Blood clots
- Poor joint mobility
- Nerve damage
- Different leg lengths
If you have a chronic illness, your risk of complications increases. For example, sleep apnea patients have a higher in-hospital mortality risk following joint replacement surgery. The risk of complication also increases with revision surgery, a second surgery required to adjust or replace an implant if it fails.
More serious complications occur in less than 2% of patients. Infection is considered one of the most serious post-surgery complications. Organisms that may enter the wound can attach to the prosthesis and may be difficult to kill with antibiotics.
Joint infection symptoms include pain and swelling, a leaking wound and fever. Depending on the type of bacteria, infections can be dealt with one of two ways: Debridement, washing out the infection, exchanging the plastic spacer and leaving the metal implants intact; and staged surgery, where the implant is completely removed.
Other serious conditions such as heart attack or stroke are rare. Complications can also arise if the implant fails and causes loosening of the joint. This may require revision surgery to replace the original components.
Revision Total Knee Replacement
While today’s total knee replacement implants are designed to last 15 to 20 years or more, eventually prostheses break or wear down. In some cases, an implant may fail sooner than expected for a variety of reasons. This can lead to pain, swelling and difficulties performing everyday activities.
In the event a knee replacement fails, the doctor may suggest revision surgery. This corrective surgery is more complicated and difficult to perform than the original surgery and usually takes two to three hours.
Special implants and tools are required. Implants used in revision surgery have thicker, longer stems to provide more stability and compensate for weaker ligaments and increased bone loss.
According to the International Survey of Primary and Revision Total Knee Replacement, the U.S. has an 8.3% national revision burden, defined as the fraction of revision surgeries among all primary and revision total knee replacements. This means that of the 600,000 annual knee replacements, more than 90% of patients experience a favorable outcome.
Implants can fail because of a patient’s active lifestyle or because of normal wear and tear. Patients who receive a new knee when they’re young and active will require revision surgery at some point, often sooner than 15 years. However, implants can also fail because of a faulty design that causes loosening and abnormal wear.
Complications arising from defective implants that may require revision surgery include:
- Loosening: Some uncemented knee implants are designed for the bone to “grow” into the space and prevent loosening. Because of poor design, the bone may not fuse properly with the implant and loosening may occur.
- Bone fracturing: Loosening of the implant can cause pain and instability, which can lead to bone fractures.
- Dislocation: Sometimes the patella dislocates after surgery, especially a patella resurfaced with a plastic button.
- Joint instability: Typically occurs when the ligaments are damaged or improperly balanced during primary surgery.
Because knee implants are regulated under the U.S. Food and Drug Administration’s 510(k) clearances program, manufacturers are not required to conduct tests on them before releasing them to the public as long as they are similar to other products already on the market. As a result, surgeons and patients may be unaware that a device isn’t working properly until it has already been implanted.
What Is the Average Cost?
The Journal of Orthopaedic Surgery and Research reports that the average cost of knee replacement surgery in the U.S. in 2023 is $29,300. While insurance usually helps cover the cost, patients may still have significant out-of-pocket costs.
Partial knee replacement is typically about 20% less expensive than full knee replacement. Revision surgery, however, can cost considerably more than primary knee replacement surgery.
Projected hospital costs for total knee revision surgeries are expected to exceed $13 billion by 2030. Revision surgery is expected to grow 500% by 2030.
Are There Alternatives?
Speak with your doctor to determine the potential effectiveness of alternatives to surgery. Your doctor may determine, however, that knee replacement surgery is necessary for you.
- Diet and Exercise
- Reducing weight can reduce the wear and tear on the knee. Losing one pound relieves as much as five pounds of stress on the knee. Muscles absorb stress on the knee. Building muscle can reduce knee pain.
- Physical Therapy
- Physical therapists help shape a routine to best build muscle. Heating, cooling and other therapies can reduce knee pain.
- Oral Medications
- Over-the-counter versions of Tylenol can help manage pain. Your doctor may write a prescription for stronger pain medication as needed.
- Knee Injections
- Cortisone shots in the knee can help ease pain and inflammation. Hyaluronic acid derivatives may also help, supplementing naturally occurring fluid in the knee that absorbs shock. Arthritis causes people to lose this natural fluid.
- Arthroscopic Surgery
- Minimally invasive surgery can remove damaged cartilage. Recovery is faster and less painful than knee replacement.
- Cooled Radio Frequency Ablation
- This procedure uses radio frequencies to “rewire the knee.” It blocks nerves that carry pain signals to the brain. Marketed as “Coolief,” it only blocks pain and does not repair damage.
- Cartilage Transplant
- One version replaces damaged cartilage with new cartilage from a donor. This method is called Missouri Osteochondral Allograft Preservation System (MOPS). Researchers at the University of Missouri developed it. Another procedure uses healthy cartilage from the patient’s own body. It moves pieces of the healthy cartilage to damaged knee. This procedure is called MACI, or Matrix-induced autologous chondrocyte implantation.
Be sure to discuss any alternative therapies you have or are considering trying with your doctor. Discuss any possible drug interactions or side effects, appropriate exercise plans to avoid further injury and the success rate of other procedures when weighing the best options for your specific health needs.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


