Yaz
Yaz is a popular oral contraceptive pill that contains a combination of drospirenone and ethinyl estradiol. Side effects of Yaz include spotting, breast tenderness and headache. Yaz is not recommended for women who have a history of headaches with aura, heart disease, blood clots or breast cancer.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Reviewed by Kevin Lombardi, M.D., MPH
- Last update: December 1, 2025
- Est. Read Time: 9 min read
What Is Yaz?
Yaz is an FDA-approved oral contraceptive that is also used to treat premenstrual dysphoric disorder (PMDD) and acne. Women may take it on its own or in combination with other treatments to produce the desired effects.
Each Yaz pill contains three milligrams of drospirenone and 0.02 milligrams of ethinyl estradiol.


Yaz is not the same as two similar-sounding medications: Yasmin and Yasminelle. All three drugs are contraceptives, but Yasmin contains more ethinyl estradiol than Yaz. Yasminelle has the same amount of ethinyl estradiol as Yaz but contains three extra placebo pills in a month’s supply.
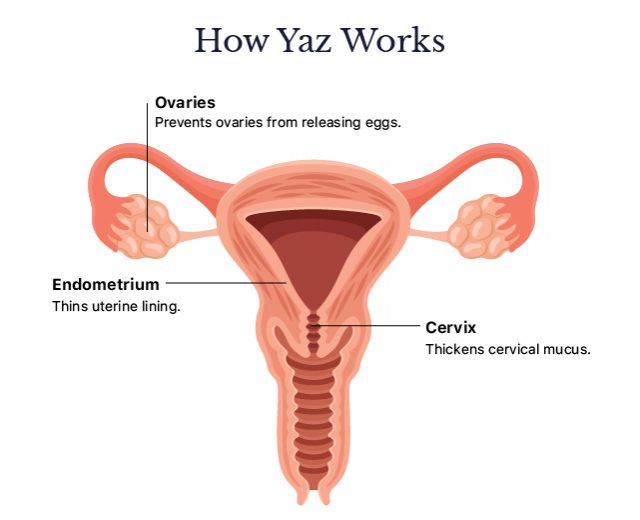
How Does Yaz Work?
Yaz prevents pregnancy in two ways:
- Hormones in the drug prevent your ovaries from releasing eggs.
- They also thicken your cervical mucus and thin your uterine lining, making it difficult for sperm to travel or for a fertilized egg to attach to your uterine wall.
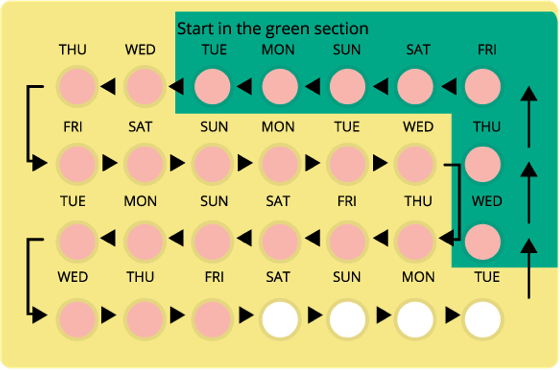
A month’s supply of Yaz includes 24 pink pills containing hormones and four white placebo pills. While taking the placebos, you’ll get your period. You may also continue taking the pink pills uninterrupted, skipping the placebos and avoiding having a period.
How To Take Yaz Birth Control Pills
You should take one Yaz pill daily at the same time each day. Follow the order of the pills in the blister pack. If you choose to take the inactive white pills, only do so when you’ve completed all of the pink pills in the pack.
You can take Yaz pills with or without food, but it’s best to take them after your evening meal or at bedtime with some liquid.
Missing an active dose of Yaz can reduce its effectiveness in preventing pregnancy. If you forget to take a dose, take the missed pill as soon as you remember.
Vomiting and severe diarrhea can also disrupt your body’s ability to absorb the drug. If you throw up within three hours of taking Yaz, consider it a missed dose.
Daily Yaz Dosage
You can start taking Yaz on the first day of your period or the first Sunday after your period starts. Take one pink pill daily for 24 days, then one white pill for four days.
The timing for taking Yaz can vary if you switch from one birth control pill or method to Yaz.
- Switching from a different birth control pill: Start taking Yaz on the same day that you would have started a new pack of your old pills.
- Switching from a different birth control method: If you are switching from a transdermal patch, vaginal ring or injection, start Yaz when the next application or dose would have been due. If you are switching from an intrauterine device or implant, start taking Yaz the day after removal.
- After pregnancy: Start taking Yaz no sooner than four weeks after giving birth to avoid an increased risk of blood clots. Do not take Yaz if you are currently pregnant or breastfeeding.
Yaz doesn’t become fully effective until you take it for at least seven days. Use an alternate form of birth control, like condoms or contraceptive sponges, while Yaz builds to full strength.
What Are the Side Effects of Yaz?
The most common side effects of Yaz are spotting or bleeding between menstrual periods, breast tenderness, nausea and headache. These side effects are usually mild and often go away.
Yaz is also less likely to make you gain weight compared to other birth control pills that are not formulated with drospirenone.
Yaz carries a black box warning stating that women who smoke cigarettes while taking the drug have an increased risk of cardiovascular events. The risk is higher for women over 35. Women who smoke should not take Yaz or any other oral contraceptive that contains estrogen.
Common Yaz Side Effects
The package insert for Yaz indicates that the most common side effects identified in clinical trials include headaches, irregular periods and nausea or vomiting. These symptoms are generally mild and resolve on their own.
People with a history of migraines with aura are advised against taking Yaz. A significant number of trial participants reported experiencing breast pain or tenderness and mood swings with Yaz.
Bayer, the manufacturer of Yaz, also lists depressive mood, vaginal discharge and fungal infection as frequent side effects. Those who take Yaz as a treatment for PMDD report side effects including fatigue, irritability, decreased libido and weight gain.
- Headache (6.7%)
- Menstrual irregularities (4.7%)
- Nausea and vomiting (4.2%)
Most birth control pill side effects resolve once you’ve taken the medication for several months. However, you should notify your health care provider about any adverse reactions so that they can be reported to the FDA.
Serious Yaz Side Effects
There are potential risks of severe adverse reactions when taking Yaz, including blood clots, gallbladder disease and elevated potassium levels. If ignored, these Yaz side effects can cause dangerous health complications or death.
Report any of these serious side effects to your health care provider immediately if you experience them while taking Yaz.
- Blood clots
- Gallbladder disease
- Hyperkalemia
High potassium levels in your blood, or hyperkalemia, may not result in symptoms but can lead to complications like weakness, sudden cardiac arrest or kidney failure.
Certain patients, including those with a history of blood clots, stroke, heart attack or kidney failure, face an increased risk of developing blood clots and other serious reactions while taking Yaz. Patients with uncontrolled blood pressure or migraine headaches with aura are also advised against taking Yaz.
Blood Clots
The package warns that some studies indicate people taking Yaz have an approximately threefold chance of developing blood clots.
Deep vein thrombosis is a type of blood clot that forms in the legs and often causes pain and swelling. If a blood clot dislodges in any part of the body, it can become stuck in the heart, lungs or brain, posing serious health risks or even death.
Bayer, Yaz’s manufacturer, agreed to pay more than $2 billion in a settlement to resolve Yaz lawsuits over its failure to warn patients adequately about the risk of blood clots. A large multidistrict litigation over this failure closed on January 2, 2019. But many women remain unaware of the increased risk of blood clots associated with oral contraceptive use.
Gallbladder Disease
Gallbladder disease is a rare but serious potential side effect of Yaz birth control pills. However, the exact cause of the risk is unknown.
Disease of the gallbladder can cause intermittent pain and may progress to include nausea, vomiting, fatigue, fever and chills. The latter symptoms require medical attention and could result in the need for surgical removal of the gallbladder.
“Long term, Yaz may increase the risk of gallstones due to fluctuations in estrogen,” Sazan Sylejmani, owner and pharmacy manager of Westmont Pharmacy in Westmont, Ill., told Drugwatch. “Studies show a small increased risk, especially in the first year of use, but the risks seem to decrease over time.”
Hyperkalemia
Patients taking Yaz face a small risk of developing high potassium levels, also known as hyperkalemia, because the active ingredient drospirenone interferes with kidney function. The FDA requires a warning of this risk on Yaz packaging.
Symptoms of hyperkalemia include an irregular heartbeat, weakness, paralysis, dizziness and fatigue. Some diuretics and medications for pain and high blood pressure may also increase the risk of kidney dysfunction and hyperkalemia.
Other Potential Yaz Side Effects
Some evidence points to an increased risk of liver disease, high blood pressure and certain types of cancers in people who take hormonal birth control pills like Yaz.
Most of the evidence linking oral contraceptives to cancers comes from observational studies that do not prove a causal relationship. However, many of these studies point to an increased risk of cervical and breast cancer with hormonal birth control use.
How To Manage or Avoid Yaz Side Effects
Most Yaz side effects resolve on their own with consistent use. You may find relief from mild pain and discomfort with over-the-counter medications. However, you should always check for the risk of interactions when taking multiple medications.
- Take your pills at the same time each day
- Drink plenty of fluids daily
- Use over-the-counter pain relievers for headaches
- Eat a bland diet to minimize nausea
Seek immediate medical attention if you experience any symptoms that might indicate a blood clot or hyperkalemia, including dizziness, extreme fatigue or swelling and pain in your legs. Left untreated, blood clots and high potassium levels can cause serious health complications and death.
Yaz Precautions
Yaz may not be the best birth control option for some women. Discuss your full medical history with your doctor before taking Yaz, especially any history of heart disease, blood clots or hormone-sensitive cancer. Taking Yaz could make these problems worse.
- Have preexisting kidney, liver or adrenal disease
- Are at high risk for arterial or venous thrombotic diseases
- Have ever had blood clots in your legs, lungs or eyes
- Have rhythm diseases of the heart, such as atrial fibrillation
- Have ever had a stroke or heart attack
- Have or had breast cancer or other estrogen- or progestin-sensitive cancer
- Have or had cholestatic jaundice of pregnancy or jaundice from other birth control pills
- Have uncontrolled high blood pressure
- Have diabetes with vascular disease
- Have migraine headaches with aura
- Are age 35 or older and smoke heavily
- Take certain combinations of drugs for hepatitis C
- Have recently had major surgery requiring bed rest
- Are experiencing undiagnosed genital bleeding
- Are pregnant
Don’t take Yaz unless your doctor says it’s safe for you. If Yaz is not an option for you, ask your physician what type of birth control fits someone with your medical history.
As of 2022, women who have experienced rare but serious adverse effects are filing Yaz lawsuits.
Yaz Interactions
Certain foods, drugs and supplements interact with Yaz and make it less effective or amplify its side effects. The medication’s absorption rate is slower when taken following high-fat meals.
- Reduced Effectiveness: Phenytoin, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampin, topiramate, barbiturates and products containing St. John's wort may decrease the effectiveness of birth control pills like Yaz.
- Raising Yaz Levels: Grapefruit juice, vitamin C supplements and foods that are very high in vitamin C may raise the level of Yaz present in your bloodstream. This is true for most birth control pills. Atorvastatin, acetaminophen, azole antifungals, verapamil, macrolides and diltiazem can also increase the amount of Yaz in the bloodstream.
- Increased Liver Enzymes: Taking hepatitis C drug combinations that include ombitasvir, paritaprevir or ritonavir at the same time as Yaz may result in increased liver enzymes.
- Increased Potassium Levels: Yaz may increase the amount of potassium in your blood. Women who take other drugs that can increase potassium levels should have their potassium levels monitored while on Yaz.
Discuss your diet and other medications with your doctor before taking Yaz. If you need to take medications with known interactions, your doctor may prescribe a different type of birth control for you.
Why Was Yaz Recalled?
Bayer recalled approximately 33,000 boxes of Yaz in 2009 because of incorrect specifications for the dose of drospirenone in the drug. The manufacturer had incorrectly claimed that the levels of drospirenone in its product fell within normal parameters.
Despite this controversy, the many Yaz settlements that were paid out, and the introduction of generic competitors to the market, sales of Yaz still generate millions of dollars in revenue for Bayer. The drug is considered safe to take as long as your doctor reviews your medical history with you first.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.





