da Vinci Robotic Surgery Lawsuits
da Vinci Surgical System lawsuits claim system malfunctions caused electrical burns, organ damage and death. Intuitive Surgical Inc. set aside $67 million to settle 3,000 cases in state and federal courts.
- Last update: April 16, 2025
Status of da Vinci Robotic Surgery Lawsuits
As of July 2024, there have been no updates in the da Vinci Surgical System litigation. Most cases were settled in April 2014, and our legal partners are not accepting new cases.
Intuitive said it set aside $67 million to settle roughly 3,000 claims related to the da Vinci Surgical System, some dating back to 2012. After extensive mediation, Intuitive said it would be more cost-effective to settle than litigate.
However, some cases have continued in state and federal courts. According to a February 2024 lawsuit filed by Harvey Sultzer, Intuitive Surgical listed about 93 da Vinci lawsuits in various courts across the country in its SEC filings.
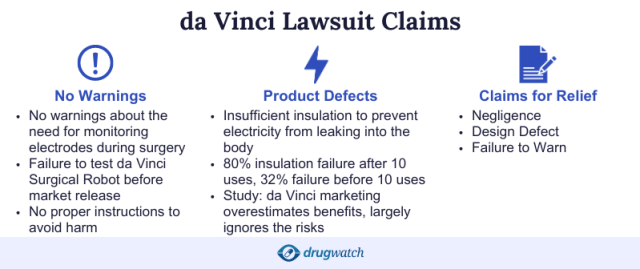
Why Did People File Lawsuits?
People filed lawsuits for various complications related to two parts of the robot: Monopolar Curved Scissors and instrument tip covers.
Plaintiffs claimed Intuitive was guilty of negligence, producing a defective product and failing to warn the public — and they demanded compensation for physical, emotional and financial damages, as well as punitive damages.
The lawsuits claimed people suffered da Vinci robotic surgery complications, including electrical burns, organ damage and death. Cases were filed in both federal and state courts.
| NAME OF PLAINTIFF | DA VINCI SURGERY PERFORMED | INJURY |
|---|---|---|
| Erin Izumi | Endometriosis surgery | Colon and rectum torn/additional procedures and temporary colostomy (a piece of the colon is diverted to an artificial opening in the abdominal wall) |
| Patricia Mayfield | Hysterectomy | Painful abscess/additional surgery |
| Kimberly McCalla | Hysterectomy | Bleeding in pelvis from laceration to main artery/death |
| Jennifer Silvestrini | Thyroid removal | Extensive scarring on neck requiring plastic surgery to repair/possibility of subsequent surgeries |
| Erika Starr | Not specified | Bowel perforation/ thermal burns/infection |
| Michelle Zarick | Hysterectomy | Bowel prolapse through vagina/massive infection with cysts and adhesions/additional open surgery/ removal of right fallopian tube |
Some of the injuries claimed in lawsuits were deadly. For example, plaintiff Harvey Sultzer’s wife, Sandra Sultzer, suffered a bowel perforation that eventually led to her death.
“Defendant knew of the da Vinci robot’s defective nature, as set forth herein, but, in conscious and or reckless disregard of the foreseeable harm caused by the robot, continued to design, manufacture, market, and sell it so as to maximize sales and profits at the expense of the health and safety of the public, including Sandra Sultzer,” according to Harvey Sultzer’s lawsuit.
“Defendant knew of the da Vinci robot’s defective nature, as set forth herein, but, in conscious and or reckless disregard of the foreseeable harm caused by the robot, continued to design, manufacture, market, and sell it so as to maximize sales and profits at the expense of the health and safety of the public.”
Examples of da Vinci Lawsuit Claims
The da Vinci cases were complex, involving design defects, a lack of training and underreporting of adverse events.
Overview of Shawn’s Claim
Shawn Todd filed her lawsuit against Intuitive after alleging she suffered internal burns while undergoing a partial hysterectomy. She was then hospitalized for weeks.
Injuries Alleged
Todd was held down following the robotic surgical procedure as needles were shoved into her failing kidneys because doctors couldn’t get anesthesia to work. Todd’s ureters (the part of the body that carries urine from the kidneys to the bladder) had been burned. Todd said the burns were caused by “arcing” from the da Vinci system.
‘Arcing’ and Insulation Failure Claims
Most plaintiffs in litigation against Intuitive complained of injuries caused by malfunctions leading to leaks of electrical currents and arcing. Arcing is when sparks from a surgical instrument land in a patient’s body, causing burns. Insulation failure is known to cause arcing when protective covers are cracked or damaged in other ways.
A surgeon would rarely be aware of arcing during a procedure. It would likely take several days for a patient to complain of symptoms and for the injury to be detected. Plaintiffs allege that they experienced perforations, infections, abscesses and other heat-related damage that required additional procedures to correct due to arcing during their procedures.
Other devices used in robot-free procedures can also cause arcing. However, a 2011 study in the American Journal of Obstetrics & Gynecology said, “Robotic instruments have a significantly higher incidence and prevalence of [insulation failure] compared with laparoscopic instruments.”
Overview of Fred’s Claim
Fred Taylor filed a personal injury lawsuit against Intuitive in 2012, alleging the company failed to warn hospitals of robotic surgery dangers, leading to insufficient training. After his death, his wife proceeded with the lawsuit, which was amended to a wrongful death lawsuit.
Injuries Alleged
Taylor’s prostate surgery took more than 13 hours. The procedure usually takes a fraction of that time. Taylor suffered from a torn rectum, leading to the loss of 15 cups of blood and shock.
He suffered a stroke, causing incontinence, kidney and lung damage and memory loss. He died four years after the surgery but as a direct result of the ongoing complications suffered from the robot-assisted procedure.
Insufficient Training Claims
Dr. Scott Bildsten, the surgeon who performed Taylor’s surgery, had performed two da Vinci surgeries on patients before Taylor’s surgery. Taylor’s da Vinci surgery was Bildsten’s first without supervision.
Bildsten stopped using robots one year after operating on Taylor. He said the “learning curve [is] so steep and lengthy that the level of comfort just took too long,” CNBC reported.
Outcome
Taylor’s family lost its lawsuit at trial and on appeal. However, they won a new trial in February 2017. That’s when the Washington Supreme Court ruled that the lower court should have told Intuitive that it had a duty to warn the hospital — not just the doctor — about the dangers of robot-assisted surgeries.
Overview of Sonya’s Claim
Some lawsuits claim reports of complications are artificially low because surgeons and patients aren’t aware of problems until days after procedures. Sonya Melton’s attorney says her case is part of the underreporting trend.
Injuries Alleged
Melton was initially unaware that her injuries may have been caused by the da Vinci system used in her minimally invasive surgical procedure. After her routine same-day gynecological procedure to remove uterine fibroids, Melton experienced intense pain and developed pneumonia. Doctors operated again, this time performing a “full-open surgery” and finding a perforation in her small intestine. Melton later sued.
Underreporting Claims
An attorney for Melton and other plaintiffs, Dr. Francois Blaudeau said problems from robot-assisted surgeries may not get reported to the FDA as an “adverse event” because sometimes symptoms develop after procedures.
da Vinci Tip Cover Recall
In 2011, Intuitive recalled its EndoWrist MCS tip cover, an insulator for a device that uses monopolar (single electrode) energy to cut tissue. In 2012, Intuitive initiated a second recall for all those who did not respond to the initial one.
- Avoiding instrument collisions.
- Carefully installing tip covers.
- Straightening wrist (of instrument) prior to removal.
- Inspecting cannula (thin tube inserted into a vein or body cavity) prior to use.
- Not exceeding power settings.
The recall was a Class II recall, meaning the device may cause temporary or treatable injuries.
Even after the recalls, Intuitive said it was “confident that the da Vinci Surgical System deploys monopolar energy in a safe and effective way when used as indicated.”
Furthermore, NBC reported that when asked about the timing of the recalls and replacement tip cover, Intuitive responded, “We are always looking at our system and our instruments to see how we can improve the safety. I wouldn’t say we changed anything based on… lawsuits.”
The FDA received 4,600 adverse events reports about the da Vinci Surgical System over 13 years ending in 2013. Nearly 1.5 million surgeries were performed during that time using the robotic system. Blaudeau, both an Alabama gynecologist and lead plaintiffs’ attorney, said it’s possible for injuries to happen without surgeons realizing.
Certain injuries resulting from the surgeries can take several days to appear.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


