Gadolinium
Gadolinium-based contrast agents (GBCAs) help doctors see abnormal tissues in magnetic resonance imaging (MRI) scans with more detail. They help doctors diagnose inflammation, tumors and blood clots by providing them with clearer, brighter images from inside the body. The U.S. Food and Drug Administration approved eight brands of gadolinium-based contrast agents, but the manufacturer of Optimark discontinued the brand in 2018.
- Medically reviewed by Jessica Elste, Pharm.D., BCPS
- Last update: March 13, 2025
Sometimes called MRI contrast media, agents or dyes, gadolinium-based contrast agents (GBCAs) provide doctors and radiologists with sharper, more accurate MRI images. This helps diagnose and monitor the progress of serious health conditions such as cancer.
During MRI scans with contrast, healthcare providers inject patients with the drugs. The active ingredient in GBCAs is a rare, silvery-white earth metal called gadolinium. It reacts with atoms and molecules in the body to make them easier to see in imaging scans.
Alone, gadolinium is toxic to humans. But gadolinium in GBCAs goes through a process called chelation, which makes it safer for use in the body. In chelation, other chemical ions mixed with gadolinium will surround the toxic metal and prevent it from harming the body while also preserving its ability to enhance contrast in tissues. Then, healthy kidneys expel the chelated gadolinium out of the body through urine before it can cause toxic reactions.
Researchers classify GCBAs into two types based on their chemical structure. Linear, or open chain, agents have chelation ions that coil around the gadolinium ions like a snake. Macrocytic agents have ions that completely enclose the gadolinium almost like a cage.
| Brand | Active Ingredient | Type |
|---|---|---|
| Dotarem | gadoterate meglumine | Macrocyclic |
| Eovist | gadoxetate disodium | Linear |
| Gadavist | gadobutrol | Macrocyclic |
| Magnevist | gadopentetate dimeglumine | Linear |
| MultiHance | gadobenate dimeglumine | Linear |
| Omniscan | gadodiamide | Linear |
| Optimark (discontinued) | gadoversetamide | Linear |
| ProHance | gadoteridol | Macrocyclic |
In 2017, the U.S. Food and Drug Administration (FDA) required a new warning on all GBCAs to alert patients that these drugs may cause gadolinium retention. This means gadolinium may remain in the body and brain for months to years. Pregnant women, children, patients with conditions that cause inflammation and those that may require numerous doses of GBCA over their lifetime are more likely to retain gadolinium. However, the FDA currently recommends that patients should proceed with MRI scans with GBCAs when needed, and states that retention of gadolinium has not directly been shown to negatively affect the health of patients with normal kidney function.
Some people have filed lawsuits over various health issues they say gadolinium retention caused. Linear agents are more likely to cause gadolinium retention and for a longer period than macrocytic agents, the FDA said in its safety communication.
Radiologists used gadolinium in about 8.8 million procedures in the United States in 2016, according to a 2018 article in JAMA by Dr. Deborah Levine and colleagues.
When Radiologists Use Gadolinium-Enhanced MRI Scans
About one in three MRIs use GBCAs to enhance parts of the body, Dr. Nick Ferris and Professor Stacy Goergen wrote in Inside Radiology. Gadolinium-enhanced scans are especially useful in detecting inflammation and any area with increased blood flow. This makes them good for diagnosing and treating cancer or inflammatory disease.
Some conditions for which radiologists may use GBACs include:
- Brain injuries
- Spinal cord injuries
- Inflammation of solid organs such as the kidney and liver
- Inflammation or cancerous cells in bone, muscle and connective tissue
- Inflammatory bowel disease
- Inflammatory joint disease
- Some types of angiograms (imaging of the blood vessels in the heart)
- Blood vessel problems
Administering Contrast Agents
Before a patient gets an MRI, the radiologist will read the referring doctor’s notes and decide whether to use a gadolinium-based contrast agent. Before the scan, patients answer questions about their medical history so medical professionals can determine if there are any risks. For example, patients with kidney problems are at higher risk for toxic reactions from gadolinium.
The radiologist then lets the patient know they will be using a GBCA. A technologist, nurse or radiologist may administer the injection by hand or through an automated injector. Patients may refuse the injection or seek another opinion.
The injection is quick and only takes about 10 to 30 seconds. Most of the time, no serious reactions occur. A few people may feel a cold sensation in the arm. In people with healthy kidneys, about 90 percent of the gadolinium leaves the body within 24 hours after the test.
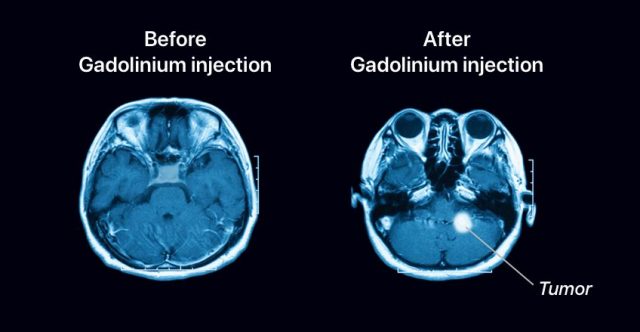
Side Effects and Warnings
In general, most people don’t have side effects from gadolinium. In clinical trials, most reactions to GBCAs were mild to moderate and usually disappeared after a few days, according to drug labels. Between one and four in 100 people may experience mild headache or nausea. Fewer than one in 100 people may vomit, according to Ferris and Goergen.
In a 2022 clinical study of nine women with five or more GBCA exposures due to screening breast MRI, there was no association between brain MRI signal changes and clinical abnormalities on neurologic or neuropsychologic examination.
All of these drugs have a black box warning for a rare condition called nephrogenic systemic fibrosis (NSF). This condition causes toughening of the skin, muscles and internal organs, and can potentially lead to death. The risk increases in people who have had multiple MRIs with gadolinium agents. Most people with kidney disease should not use gadolinium-based contrast agents.
“As concern about gadolinium-based contrast continues, it is going to be important that other options can be advanced and approved for medical imaging.”
Concerns over gadolinium-based contrast agents have researchers looking for safer alternatives. Researcher Shumin Wang, director of the National Institute of Biomedical Imaging and Bioengineering (NIBIB) program in Magnetic Resonance Imaging, said finding a compound better filtered by the body would reassure patients and doctors.
“As concern about gadolinium-based contrast continues, it is going to be important that other options can be advanced and approved for medical imaging,” Wang said in a National Institute of Health press release.
GBCAs are generally safer than iodine-based agents, according Levine and colleagues. The authors wrote that the rate of acute side effects for GBCAs was 0.07 percent to 2.4 percent, which is lower than the rate seen when iodine-based agents are used. According to the drug’s labels, GBCAs can pass from pregnant women to their fetuses, but there are no studies on how this affects babies.
- Headache
- Nausea
- Dizziness
- Injection site reactions such as pain, irritation, burning, or a cold sensation
- Altered taste (dysgeusia)
- Feeling warm
In addition to these less serious side effects, GBCAs are associated with some rare but serious health issues. These come from postmarketing reports outside of clinical trials. According to the drugs’ labels these reactions cannot be definitely linked to gadolinium-based contrast agents. Some reported conditions include: Nephrogenic Systemic Fibrosis, irregular heartbeat, mild to severe allergic reactions, damage to the kidneys, skin plaques and pain syndromes.
Brands
The FDA approved the first GBCA, Bayer’s Magnevist (gadopentetate dimeglumine), in 1988. The agency went on to approve seven other agents for use in the United States. Though, manufacturing of Optimark has since been discontinued. The majority of brands available are linear, which means they have a higher rate of gadolinium retention.
The American College of Radiology (ACR) divides the different brands of GBCAs into three groups based on their associated risk of Nephrogenic Systemic Fibrosis. Omniscan and Magnevist, and Optimark, for example, make up Group I. They have the greatest number of NSF cases, according to the American College of Radiology.
These classifications include:
- Group I Agents – have the greatest number of NSF cases
- Group II Agents – associated with few, if any, confirmed cases of NSF
- Group III Agents – data is limited about NSF risk
Dotarem
Dotarem (gadoterate meglumine injection) is a macrocyclic contrast agent. Guerbet LLC manufacturers the drug, which first hit the market in 2013. It is FDA-approved for MRI imaging of the brain, spine and associated tissues in adults and children including newborns.
- Dosage: The recommended dose is 0.2 mL/kg body weight through manual injection or power injector at a rate of 2 mL a second for adults and 1 mL to 2 mL a second for children.
- Interactions: Drug interactions have not been studied with Dotarem.
- Risk of NSF: Group II
Eovist
Eovist (gadoxetate disodium) is a linear contrast agent manufactured by Bayer. The FDA approved Eovist in 2008 for use in MRIs of the liver to detect lesions in people with suspected liver disease that is localized to one area of the liver. Researchers have not conducted adequate studies to determine the safety and effectiveness in pediatric patients.
- Dosage: The recommended dose of Eovist is 0.1 mL/kg of body weight at a rate of 2 mL a second in an injection. Imaging can begin 15 to 25 seconds after Eovist administration.
- Interactions: None listed in the drug label.
- Risk of NSF: Group III
Gadavist
Gadavist (gadobutrol) is a macrocylic contrast agent manufactured by Bayer. The FDA first approved the drug in 2011 for use in MRIs in adults and children to detect abnormalities and visualize areas within the central nervous system. The drug is also approved to assess breast cancer, evaluate known or suspected artery disease in the heart or kidneys and assess blood flow to the heart in patients with known or suspected heart disease.
- Dosage: Bayer’s recommended dose of Gadavist is 0.1 mL/kg of body weight in children and adults. This drug’s formula is more concentrated than other GBCAs, so healthcare providers administer it at a lower volume. The flow rate differs depending on the body part the radiologist is studying.
- Interactions: None listed on drug label.
- Risk of NSF: Group II
Magnevist
Magnevist (gadopentetate dimeglumine injection) is a linear contrast agent manufactured by Bayer and approved in 1988. It was the first GBCA approved in the United States. The FDA approved Magnevist for adults and children 2 years of age or older for MRIs of the central nervous system, tissues in the head and neck, and the body.
- Dosage: Bayer’s recommended dose of Magnevist is 0.2 mL/kg body weight. Healthcare providers should not administer it faster than 10 mL/kg per 15 seconds.
- Interactions: No known listed interactions.
- Risk of NSF: Group I
MultiHance
MultiHance (gadobenate dimeglumine injection) is a linear contrast agent manufactured by Bracco Diagnostics Inc. and originally approved in 2004. The FDA approved the drug to visualize lesions in the brain, spine and associated tissues in children, including newborns, and adults. MultiHance is also approved in adults and for visualizing known or suspected blockages in blood vessels of the kidney or heart.
- Dosage: The recommended dose is 0.2 mL/kg for adults and 0.1 to 0.2 mL/kg for children under 2.
- Interactions: MultiHance may prolong the exposure of the following drugs: cisplatin, doxorubicin, daunorubicin, vincristine, methotrexate, etoposide, tamoxifen and paclitaxel. Some patients may experience a rare, harmless disorder called Dubin-Johnson syndrome, which causes the liver to turn black.
- Risk of NSF: Group II
Omniscan
Omniscan (gadodiamide injection) is a linear contrast agent manufactured by GE Healthcare Inc. The FDA approved the drug in 1993 to help doctors see lesions in the brain, spine, thoracic cavity, abdominal cavity and space behind the abdominal cavity and the pelvic cavity.
It is not for use in children younger than 2 years old. In addition to the black box warning for Nephrogenic Systemic Fibrosis, Omniscan also has a boxed warning that says the drug is not for direct injection into the spine because it can cause convulsions (uncontrolled muscle contractions), coma, and other negative effects in the central nervous system.
- Dosage: Dosages range from 0.1 to 0.2 mL/kg by weight depending on age and location of the scan. Doses are given as a rapid injection.
- Interactions: No specific drug interaction studies have been conducted.
- Risk of NSF: Group I
Optimark
Optimark (gadoversetamide injection) is a linear contrast agent. Its manufacturer discontinued the brand in 2018. The FDA had approved it in 1999 for use in the brain, spine and liver.
- Dosage: The recommended dose was 0.2 mL/kg by weight at a rate of 1 to 2 mL/sec.
- Interactions: No drug interactions were studied.
- Risk of NSF: Group I
ProHance
ProHance (gadoteridol injection) is a macrocyclic contrast agent manufactured by Bracco Diagnostics Inc. The FDA approved it in 1992 to help doctors see lesions in the brain and spine in adults and children at least 2 years old, and to see lesions in the head and neck in adults.
- Dosage: The recommended dose is 0.2 mL/kg by weight in adults, which can be given by rapid injection or infusion. If scans are poor and the patient has healthy kidneys, health care providers may add a supplementary dose of 0.4 mL/kg up to 30 minutes after the first dose. The dose is the same in children, but the safety and efficacy of repeat doses has not been tested.
- Interactions: No drug interactions listed on label.
- Risk of NSF: Group II
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.