Infuse Bone Graft Lawsuits
Infuse Bone Graft lawsuits claim the product led to spinal injuries and additional surgeries. In 2014, the company settled 950 claims for about $22 million, with 3,800 pending claims outstanding. In June 2017, the company reported it set aside $300 million and resolved the remaining 6,000 Infuse lawsuits for undisclosed amounts.
- Legally reviewed by Christopher Edison, Esquire
- Last update: May 1, 2025
Infuse Bone Graft Lawsuit Overview
People who sued Medtronic claimed the company pushed doctors to use its Infuse Bone Graft for spinal fusion surgery in the neck or cervical spine. Plaintiffs claimed that Medtronic marketed the graft for off-label cervical spine fusion surgery while knowing the procedure could cause serious complications.
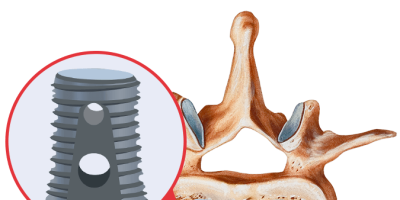
Infuse contains recombinant human bone morphogenetic protein-2 (rhBMP-2), a protein that helps regrow bone. It’s FDA-approved for lower back lumbar fusion surgery but not for use in the neck. It also has FDA approval for specific dental and trauma indications.
The Infuse Bone Graft system comes with the human bone morphogenetic protein and a scaffold or cage for the protein, and it must be used with both components. During an Infuse spinal procedure, doctors remove the disk space contents of the patient’s damaged disk. They then implant the bone graft and a cage around the graft to keep it stabilized as it heals. Infuse encourages new bone tissue growth at the site of implantation.
Most of this litigation is no longer active, and Drugwatch doesn’t know of any attorneys still taking cases. Drugwatch’s legal partners are no longer taking these cases, and we are only updating this page to provide information on this litigation for the general public.
Injuries Named in Infuse Lawsuits
- Bone migration
- Bone reabsorption
- Pain
- Seroma (pocket of excess fluid)
- Swelling of the neck and throat
- Unwanted bone growth
These serious complications can lead to more severe health problems.
- Airway compression
- Compression of nerves in the neck
- Death
- Difficulty swallowing
- Difficulty breathing and speaking
- Paralysis
These serious complications may require emergency treatment, surgery or the insertion of feeding tubes.
- Some Causes of Action
- Breach of Warranty
- Negligence
- Strict Liability
- Serious Injuries
- Infuse was never tested or FDA-approved for use in the cervical spine
- Risks of Infuse cervical spine use include airway compression and nerve damage that may lead to paralysis and even death
- Off-Label Use
- FDA reported life-threatening complications with off-label use of Infuse in the cervical spine
- Medtronic encouraged doctors to use Infuse in off-label cervical spine procedures
- Medtronic paid doctors to promote off-label cervical spine fusions
Infuse Lawsuit Updates
According to Medtronic’s 2017 annual report, the company had resolved 6,000 Infuse lawsuits. It didn’t provide a specific settlement amount, though the report shows Medtronic set aside $300 million to cover “certain litigation charges.”
The 2017 settlement of the last batch of cases was the end of this litigation, and Drugwatch isn’t aware of any lawyers taking Infuse lawsuits as of July 2025.
Recent Infuse Lawsuit Highlights
-
September 2024
The Federal Court of Australia ordered Medtronic Australasia Pty Ltd to pay $22 million for unlawfully selling over 16,000 Infuse Bone Graft Kits between Sept. 1, 2015, and Jan. 20, 2020. Australia’s TGA originally sued Medtronic in 2021.
-
August 2021
Australia’s Therapeutic Goods Administration sued Medtronic for selling Infuse without the cage component since it never approved Infuse for use without the cage. While this lawsuit is unrelated to Infuse Bone Graft injury claims in the U.S., it highlights similar litigation in Australia over Medtronic’s alleged wrongdoing and deceptive sales practices.
-
December 2017
Medtronic paid $12 million to settle a lawsuit filed by five states’ attorneys general claiming the company had deceptively marketed Infuse. According to the lawsuit, Medtronic paid millions in consulting fees to doctors to publish studies downplaying Infuse’s side effects and failed to discuss any adverse results.
-
May 2014
Medtronic announced it settled 950 Infuse claims for $22 million, though the company denied liability. “This agreement is a compromise of disputed claims and is not in any way an admission of liability or validity of any defense in the litigation by Medtronic,” stated the company in a press release.
-
March 2012
Medtronic paid $85 million to settle a shareholder lawsuit in 2012 for concealing that most Infuse sales were for off-label uses. The lawsuit claimed Medtronic failed to reveal that as much as 85.2% of Infuse sales came from off-label uses.
The U.S. Department of Justice also investigated Medtronic. The investigation ended in a $40 million payout to settle allegations of illegal kickbacks to doctors as incentives to use Medtronic’s Infuse product in 2006. In these types of cases, kickbacks are typically financial bribes from a medical company to incentivize doctors to recommend their products to other providers and to use them on their patients. Allegedly, Medtronic paid consulting fees to doctors to publish studies that downplay the risks of Infuse.
People Who Sued Over Infuse Bone Grafts
Patients have filed thousands of lawsuits claiming Infuse Bone Graft has caused complications ranging from unwanted bone growth to death. Lawsuits named Medtronic, the hospitals where plaintiffs’ spinal surgeries were performed and/or the surgeons who performed their procedures.
Case Study: Lori Byrnes
Off-label use of Infuse Bone Graft in spinal fusion surgery has resulted in legal claims of negligence and fraud. In addition, plaintiffs have made economic and noneconomic claims, including loss of consortium (refers to loss to benefits of a familial relationship).
Severe and continuous pain, bony overgrowth and economic losses.
Medtronic and the plaintiff agreed on a confidential settlement, and the case was eventually dismissed.
Karl Sanda’s Infuse Bone Graft Story
Karl Sanda’s doctor, Mark T. Nolden, diagnosed him with cervical spondylosis and degenerative cervical stenosis in the vertebrae in his cervical spine. He recommended spinal fusion surgery to alleviate chronic pain and other neck problems. However, Sanda’s doctor didn’t tell him he would use the Infuse Bone Graft off-label during the surgery.
No one told Sanda that off-label use came with serious complications, like seroma (a pocket of fluid), chronic neck pain, throat swelling and more. He wasn’t informed these side effects could be life-threatening and lead to permanent injuries.
A few days after the surgery, Sanda developed a life-threatening seroma that required emergency surgery. While the seroma was removed, Sanda was left partially paralyzed with permanent disability and pain. He never recovered from the surgeries and still has “daily severe disabling pain and paralysis.”
Sanda filed an Infuse Bone Graft lawsuit in 2013. The resolution for his case was never publicized.
UCLA Hospital Pays $8.45M Settlement with Infuse Patients
Two patients who received Infuse bone grafts at the University of California Los Angeles later sued the teaching hospital, surgeon Jeffrey Wang and Medtronic, for alleged injuries resulting from the off-label use of the product.
The plaintiffs, Ralph Weiss and Jerome Lew alleged that Medtronic’s Infuse product was used in ways not approved by the FDA in both surgeries.
They further alleged that Wang failed to inform them of a financial relationship between the surgeon and the product manufacturer. The lawsuits claimed that Wang received hundreds of thousands of dollars in lectures, consulting work and royalties from Medtronic.
The University of California Los Angeles settled with Weiss and Lew for a combined $8.45 million, with Weiss receiving a little more than $4.25 million. Medtronic also settled with Lew for an undisclosed amount, but the judge dropped Medtronic as a defendant in Weiss’ case.
Editor Lindsay Donaldson contributed to this article.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.