Abiomed Impella Heart Pump Lawsuit
Abiomed Impella heart pump lawsuits claim the device is defective and caused serious injuries or death. Abiomed recalled the device because it could break during an operation, leading to reduced blood flow or total pump stoppage. Litigation is in the early stages.
See If You Qualify for an Abiomed Impella Heart Pump Lawsuit
If you were seriously injured by the Abiomed Impella heart pump or lost a loved one who was seriously injured by the defective device, you may be entitled to compensation.
- A+BBB Rating
- 4.9 StarGoogle Reviews
- A+BBB Rating
- 4.9 StarGoogle Reviews
- Last update: June 12, 2025
Latest Abiomed Impella Heart Pump Lawsuit Updates
Severely injured patients and the loved ones of those who died from a defective Impella heart pump medical device are filing lawsuits against the manufacturer, Abiomed. These filings are still in the early stages as patients and families consult with experts in product liability and wrongful death cases.
The Abiomed Impella heart pump has caused four deaths as of July 2023. Current lawsuits state that the manufacturer and sellers endangered customers and have a legal responsibility for the harm the defective devices caused.
- May 2025: A new Abiomed heart pump lawsuit has been filed that is seeking class action status for these cases. The lawsuit, which was filed in the Northern District of Ohio, claims that a medical professional says that a man’s perforated left ventricle and eventual death was probably caused by the device.
- February 2025: On February 13, Abiomed filed an Opposition to Plaintiff’s Renewed Motion for Leave to Amend Complaint. Plaintiff Christopher Urquhart wanted to amend his complaint on behalf of his wife to add new details of her injury. Urquhart claimed his wife Nancy June died because of hemolysis, the destruction of red blood cells, after using the Impella Pump. Abiomed said that the amended complaint would not prove her case that Abiomed didn’t properly add warnings to the Impella label. Abiomed claimed that the label already warns about hemolysis. If the judge agrees, Urquhart may not be able to continue with his wrongful death claim. It could also affect other claims against Abiomed for hemolysis.
- October 2024: No major updates over the last few months, as litigation is still in the early stages. Lawyers continue to accept new cases.
- May 2024: Litigation remained in the early stages, and no trials have been scheduled. This month, Abdiomed sent an Urgent Medical Device Recall letter to its customers for certain Impella CP with SmartAssist devices after nine pumps in a single lot failed inspection. These pumps may unexpectedly stop or release potentially harmful particles.
- December 2023: Lawyers continue to investigate lawsuits. So far, there have been no global settlements or trials scheduled.
- October 2023: FDA announced it sent a warning letter to Abiomed saying the company's responses to the FDA's inspection of its manufacturing facility in Massachusetts were inadequate. The FDA determined the company has been marketing its Impella Connect system without proper premarket approval.
- September 2023: Injured patients or loved ones of those severely injured or who died are currently working with lawyers to file lawsuits over the recalled pump.
- July 2023: The FDA classified the Abiomed Impella blood pump recall as a Class I recall. So far, there are 30 complaints, 26 injuries, and four deaths reported.
- June 2023: Abiomed recalled Impella blood pump devices distributed from May 1, 2021, to present day.
- March 2018: Abiomed paid a settlement of $3.1 million for allegations that it purchased expensive meals for physicians in an effort to get them to purchase its pumps.
The current Abiomed heart pump lawsuits differ greatly from the 2018 settlement. Past lawsuits were for claims that the manufacturer wrongfully tried to influence physicians to buy its products. The current developing lawsuits focus on injuries and wrongful death claims directly caused by the defective medical device.
Why Are People Filing Impella Heart Pump Lawsuits?
Patients and family members of those who underwent transcatheter aortic valve replacement are suing Abiomed over claims the Impella left sided blood pumps are causing serious injuries or even death.
Lawsuits claim the manufacturer failed to warn doctors that the pumps could malfunction and fracture during the TAVR procedure.
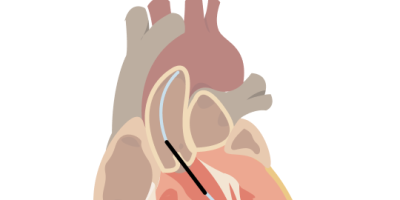
“The damaged Impella system may have reduced blood flow or pump stop, which may delay therapy or fail to provide enough support to the patient. This could be life threatening in people who require high levels of support. There is also a risk that pieces of the broken blades could enter the patient’s bloodstream.”
Many people have filed product liability lawsuits for Impella heart pump complications such as failure and other serious injuries, including brain damage, that resulted from lack of blood flow.
Some patients needed open-heart surgery to remove the device or fractured pieces of it. Family members of loved ones who died as a result of the Impella device are filing wrongful death cases.
Who Can File an Abiomed Impella Heart Pump Lawsuit?
People who were severely injured as a result of the Abiomed Impella heart pump and those who lost a loved one or whose loved one was severely injured by a defective device can file a lawsuit. An experienced product liability or wrongful death attorney can review the specific details of your case to determine eligibility.
- Plaintiff had a heart valve replacement surgery in which the doctor stated the pump failed or became damaged during the procedure.
- Plaintiff suffered brain damage from lack of blood flow or another serious injury, or had a loved one die.
- People may also be eligible if they underwent an operation during a heart attack or cardiogenic shock that used a temporary blood pump.
Wrongful death claims citing product liability must prove that the heart pump was dangerous because it was defective, either as the result of a manufacturing issue, design flaw or inadequate instructions conveying any necessary warnings.
The claim also needs to show that the defect caused the patient’s death. The main defect of the Abiomed Impella heart pump is a damaged purge sidearm, which increases the risk of purge fluid leakage.
Status of Abiomed Impella Heart Pump Recalls
The Abiomed Impella heart pump recall is a Class 1 recall, initiated on June 14, 2023. The FDA identifies this as the most serious type of recall because defective medical devices can cause serious injuries or death. The recall includes more than 7,895 units of the pump, distributed from May 1, 2021, to present day.
- December 12, 2024: Abiomed recalled one lot of its Abiomed Impella CP with SmartAssist because the lot (1798046) failed inspection but was sent to customers anyway.
- May 31, 2024: Abiomed recalled one lot of its Abiomed Impella CP with SmartAssist because the lot (1798046) failed inspection but was sent to customers anyway.
- June 29, 2023: The Impella RP Flex with SmartAssist, distributed from Nov. 1, 2022, to the present, is recalled.
- April 17, 2023: Impella 5.5 with SmartAssist devices distributed between Sept. 28, 2021, and March 6, 2023, are recalled.
Abiomed advises that doctors should not use the specified device in any operation unless an alternative is unavailable. In these cases, the company provides steps to follow to limit the risk of the pump causing injury or death.
According to the FDA recall notification’s actions for customers, the pump is “not being removed from the field and does not need to be returned.”
How to File an Abiomed Heart Pump Lawsuit
The first step in filing an Abiomed Impella heart pump lawsuit is to contact an experienced product liability lawyer. Lawsuits may be filed by injured patients or family members filing on behalf of a patient or their estate.
- Begin the legal process by consulting an experienced product liability lawyer.
- Provide the lawyer with details and documentation of you or your loved one’s medical history, including the timeline of relevant events.
- The law firm will assist you in gathering all the documents needed to review your case.
- Next, your law firm will determine if you have a viable case and assess the best approach for filing a lawsuit.
- Your lawyer will file the claim on your behalf and guide you through the process.
The compensation the plaintiff may receive from a successful lawsuit or settlement depends on the type of injuries they sustained and the severity. Damages may include past and future medical treatment, loss of income, and pain and suffering.
Proving the claims that a medical device was defective and responsible for causing an injury or death is a complicated process. Plaintiffs also need to show that the manufacturer was negligent in patient safety when distributing a faulty device. It’s critical that you retain a lawyer who is familiar with defective medical device cases.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.