Tylenol ADHD & Autism Lawsuits
Tylenol lawsuits claim the companies that manufacture and sell Tylenol failed to warn of the increased risks of autism and ADHD in children whose mothers took Tylenol. The MDL judge granted summary judgement in August 2024, but plaintiffs are appealing.
- Last update: July 1, 2025
Why Are People Suing Over Tylenol?
People are filing Tylenol lawsuits because studies have linked Tylenol or acetaminophen products taken during pregnancy to an increased risk of autism spectrum disorder, also known as ASD, and attention-deficit/hyperactivity disorder, or ADHD, in children.
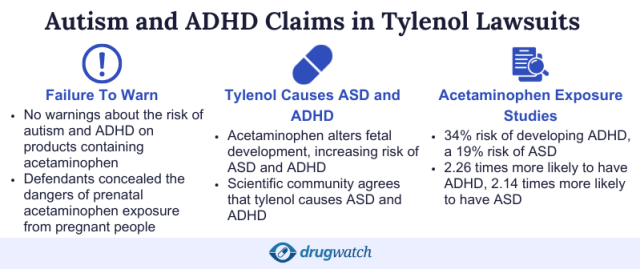
“Increasing experimental and epidemiological research shows that prenatal exposure to [acetaminophen] alters fetal development, which significantly increases the risks of neurodevelopmental disorders, including but not limited to, ASD and ADHD,” Tiffany Rutledge’s lawsuit states.
“Increasing experimental and epidemiological research shows that prenatal exposure to [acetaminophen] alters fetal development, which significantly increases the risks of neurodevelopmental disorders, including but not limited to, ASD and ADHD.”
According to our review of these lawsuits, people are filing Tylenol claims against Johnson & Johnson and various stores that sold generic acetaminophen products, including CVS, Costco, Family Dollar, Rite Aid, Safeway, Sam’s Warehouse, Target, Walgreens, Walmart and others.
Tylenol Linked to ASD and ADHD in Lawsuits
We’ve found that lawsuits claim several studies link acetaminophen to ADHD and ASD. One observational study published in the American Journal of Epidemiology found children born to women exposed to acetaminophen during pregnancy had a 34% risk of developing ADHD, a 19% risk of ASD and a 24% chance of hyperactivity symptoms.
- Scientific evidence was available, and Johnson & Johnson knew or should have known that acetaminophen in Tylenol could increase the risk of autism spectrum disorder and ADHD.
- Manufacturers should have warned the public about the risks of taking Tylenol during pregnancy.
- Tylenol and acetaminophen are marketed as safe to take during pregnancy even if evidence exists that they increase the risk of ASD and ADHD.
A study funded by the National Institutes of Health and conducted by researchers from Johns Hopkins analyzed acetaminophen levels in umbilical cord blood samples from 996 births. Researchers divided the children into three groups based on the amount of acetaminophen in the blood samples, with the third group having the highest levels.
Compared to children in the first group, “children in the middle third group were about 2.26 times more likely to have an ADHD diagnosis and 2.14 times more likely to have an autism spectrum disorder diagnosis,” according to the study.
Do I Qualify for a Tylenol Autism or ADHD Lawsuit?
According to our legal partners, you may file a Tylenol autism or ADHD lawsuit if you or your child were diagnosed with ADHD and/or autism spectrum disorder after exposure to acetaminophen in the womb. Exposure should have taken place during the second or third trimester of pregnancy.
Only a Tylenol autism lawsuit lawyer can tell you if you qualify, so we recommend you contact an attorney as soon as possible to preserve your right to file a lawsuit. Lawyers will ask you what year you or your baby were born and how long you took Tylenol while pregnant or were exposed to acetaminophen in the womb, according to our legal partners.
Make sure to have medical records with an ADHD and/or autism spectrum disorder diagnosis with you when you speak to an attorney. Proof that you took Tylenol or generic acetaminophen is also helpful evidence.
Tylenol ADHD Lawsuit Case Study
We’ve analyzed the acetaminophen, autism and ADHD lawsuit Tiffany Rutledge filed against Walmart in June 2022, on behalf of her minor daughters, L.R. and C.R.
Rutledge took acetaminophen throughout both pregnancies.
C.R. developed ADHD, while L.R. has ADHD and is being tested for autism.
Rutledge is suing for compensation for “permanent injuries and significant pain and suffering, emotional distress, lost wages and earning capacity, and diminished quality of life.”
Latest Tylenol Lawsuit Updates
As of July 2025, MDL Judge Denise L. Cote has granted summary judgement in favor of the defendants. Plaintiffs have appealed the decision.
We’ve researched court documents and spoken to legal experts to provide you with the following updates. We’ll continue to update this timeline with any key developments.
Tylenol Lawsuit Timeline
-
April 2025
We continue to await a decision on plaintiffs' appeal of Judge Cote's decision to grant summary judgement to the defendants. Lawyers do continue to investigate and accept new Tylenol lawsuits.
-
December 2024
It is expected that the Second Circuit Court of Appeals will not rule on plaintiffs’ appeal until 2025. Based on our research, it appears that while the federal MDL appeal is pending, families are filing suits in state courts around the U.S.
-
September 2024
Plaintiffs have filed an appeal of Judge Cote’s decision from August to grant summary judgement to the defendants. We will closely monitor the progress of this appeal, which was filed in the Second Circuit Court of Appeals and provide the latest updates.
-
August 2024
In a major blow for plaintiffs, Judge Denise L. Cote has granted summary judgement in the favor of the defendants. The decision was made over the ongoing issues relating to the sufficiency of plaintiff’s scientific evidence regarding causation. In her decision, Judge Cote held that plaintiffs had failed to show (through their scientific experts) that Tylenol use during pregnancy actually causes autism in babies.
-
July 2024
We are waiting to see whether Judge Denise L. Cote will grant the defendants’ motion to exclude the expert opinions of Dr. Roberta Ness. We’ve looked at the MDL docket, and the judge hasn’t ruled whether this testimony is admissible. Based on our experience reporting on mass torts, this testimony is an important part of whether litigation can continue. The MDL remains open.
-
May 2024
The MDL remained open and had not been officially closed. The plaintiffs whose cases were dismissed because of a lack of experts filed appeals. But, according to our research, after Judge Cote allowed some cases to proceed under the expert testimony of Dr. Roberta Ness, defendants filed motions to exclude these medical opinions.
-
March 2024
In a win for plaintiffs, Judge Cote signed an order that allows plaintiffs who filed their cases against certain defendants on or after Jan. 12, 2024, to proceed with their cases with Dr. Roberta Hess’s testimony.
-
February 2024
Judge Cote dismissed nearly all the claims in the New York MDL, according to an order we reviewed. We theorize plaintiffs’ lawyers will appeal this decision.
-
December 2023
A settlement conference was set for Jan. 4, 2024, to address pending challenges to the admissibility of expert testimony linking Tylenol to autism and ADHD. Judge Cote ruled to bar expert witnesses from testifying that taking Tylenol during pregnancy can lead to autism in babies. The judge found that these experts failed to support their claims with scientific evidence.
This decision will likely mark the end of the federal multidistrict litigation, which consisted of approximately 500 lawsuits involving the popular over-the-counter drug unless the plaintiffs successfully appeal the ruling. -
June 2023
More defendants were added to the cases, including 7-Eleven, Family Dollar and Dollar Tree.
-
May 2023
About 118 Tylenol autism/ADHD lawsuits were pending in federal court.
-
April 2023
Judge Cote denied Johnson & Johnson’s motion to dismiss based on preemption.
-
January 2023
Judge Cote appointed Randy Ellis as Census Special Master to better coordinate the collection of information from plaintiffs. The judge also outlined the discovery plan.
-
November 2022
Judge Cote approved the members of the Retailer Liaison Committee that will represent the defendants. The judge also denied Walmart’s motion to dismiss.
-
October 2022
Defendants CVS, Costco and Walgreens filed a motion to stay proceedings, and Judge Cote denied it.
-
October 2022
Judges transferred cases from all over the country into multidistrict litigation in New York.
While summary judgement was granted by the MDL judge, plaintiffs are still hoping to appeal the decision. If the appeal is unsuccessful, then this is likely the end of federal litigation over claims that Tylenol is linked to ADHD and autism. Lawsuits may continue in state courts.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.





