Penumbra Jet 7 Xtra Flex Catheter: Side Effects, Recall & Lawsuit Information
Penumbra recalled its Jet 7 Xtra Flex Catheter after reports of device failure leading to severe injury and death. Patients harmed by the catheter have filed lawsuits alleging manufacturing defects and a failure to warn.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Last update: November 24, 2025
- Est. Read Time: 3 min read
Why Did Penumbra Recall the Jet 7 Xtra Flex Catheter?
On December 15, 2020, Penumbra, Inc. issued a recall for its Jet 7 Xtra Flex Catheter, a device used to remove blood clots in patients who’ve experienced a stroke. Penumbra’s recall came after it issued a warning letter about potential injuries in July 2020.
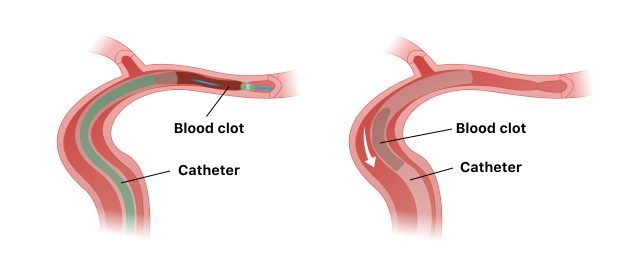
Penumbra recalled the device because the tip of the catheter may become damaged during use, especially under pressure or while injecting dye to help doctors see vessels and veins. This could cause blood vessel injuries or complications that could lead to death.
Penumbra recalled over 30,000 devices.
How Does the Jet 7 Catheter Work?
The Jet 7 Xtra Flex Catheter is a large, single-use, flexible catheter with a guide wire. It attaches to plastic tubing and an aspiration pump. The pump acts like a vacuum. The Xtra Flex technology was designed as an advancement to the Penumbra Jet 7 Reperfusion Catheter with Standard Tip.
Doctors insert the catheter through an artery in the groin, up through the neck and to the location of the blood clot causing the stroke. They use fluoroscopy, or continuous X-ray, to monitor the progress of the catheter as it travels through the artery.
Once the catheter reaches the blood clot, it sucks out the clot. This unblocks the blood vessel and restores blood flow. Doctors then guide the catheter back out of the body.
Injuries and Complications Linked to the Jet 7 Catheter
Patients and medical facilities reported numerous serious adverse effects. Some of the major complications include blood vessel damage, brain tissue damage and strokes, internal hemorrhaging and premature death.
- Acute occlusion (blockage)
- Air embolism (artery blockage)
- Allergic reaction and anaphylaxis from contrast media
- Arteriovenous fistula (abnormal connection between artery and vein)
- Bruising or hemorrhage at access site
- Cancer from X-ray exposure
- Death
- Device malfunction
- Dissection or perforation of blood vessels
- Distal embolization (blockage in a coronary artery because of a clot, broken guidewire or air)
- Emboli (blood clot, air bubble, fatty deposit or other object that can lodge in a blood vessel)
- False aneurysm formation (leaking blood in tissues surrounding an injured blood vessel)
- Inability to completely remove blood clot
- Infection
- Intracranial hemorrhage
- Ischemia (inadequate blood supply to a part of the body or organ)
- Kidney damage from contrast media used in fluoroscopy
- Neurological deficits, including stroke
- Radiation exposure that may lead to cataracts, skin reddening, burns or alopecia
- Thrombosis (blood clot)
- Vessel spasm
FDA and Safety Actions
In June 2019, the U.S. Food and Drug Administration (FDA) approved the Penumbra Jet 7 Xtra Flex Catheter through the 510(k) pre-market notification process. 510(k) is a fast-tracked process that approves medical devices that the FDA considers highly similar to existing devices.
In July 2020, Penumbra issued a warning to health care providers, advising them not to inject contrast using the device. The process was designed to inject a dye into the bloodstream to allow doctors to see veins and arteries more clearly on scans.
Penumbra instead recommended using the guide catheter. In August 2020, the FDA approved a label change to include this warning.
Then, in December 2020, Penumbra announced the urgent voluntary recall. The FDA terminated the recall on May 14, 2024.
While specific clinical trial information regarding the Jet 7 is not publicly available, the device’s recall has influenced broader discussions about safety and regulation of 510(k)-cleared devices.
Penumbra Jet 7 Lawsuits
Attorneys have filed Jet 7 lawsuits on behalf of patients and families, alleging injuries or deaths due to the device’s failure. These lawsuits allege that Penumbra manufactured defective devices and failed to warn patients about the risks.
These are typically product liability cases and can proceed as mass torts or multidistrict litigation (MDL), which groups similar cases together to streamline the legal process. Plaintiffs have argued that Penumbra knew or should have known of the risks and waited too long to recall the Jet 7 Xtra Flex.
As of September 2025, Drugwatch is unaware of any settlements or trials for Penumbra Jet 7 Xtra Flex Catheter lawsuits.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.