Saxenda (Liraglutide) Lawsuit
Saxenda (liraglutide) lawsuits claim that liraglutide-containing drugs increase the risk of non-arteritic anterior ischemic optic neuropathy (NAION), a condition that affects vision. People diagnosed with NAION after taking Saxenda may qualify for compensation.
GLP-1 NAION Lawsuit
Used Ozempic, Wegovy, Saxenda or Victoza and later diagnosed with non-arteritic anterior ischemic optic neuropathy (NAION)? You may qualify for compensation. Check eligibility windows and request a no-cost case review.
- A+BBB Rating
- 4.9 StarGoogle Reviews
- A+BBB Rating
- 4.9 StarGoogle Reviews
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Brian Ranger Wilson, Esquire
- Last update: February 22, 2026
- Est. Read Time: 4 min read
What’s the Status of the Saxenda (Liraglutide) NAION Lawsuit?
Saxenda lawsuits are being filed against drugmaker Novo Nordisk, claiming that the GLP-1 (glucagon-like peptide-1) drug caused sudden and irreversible vision loss known as non-arteritic anterior ischemic optic neuropathy (NAION).
Lawsuits also allege that the manufacturer didn’t adequately warn patients about this risk.
Similar lawsuits have been filed against GLP-1 drugs Ozempic and Wegovy. These cases have recently undergone consolidation, which is a major step when many similar lawsuits are filed. Many state cases have been grouped together in multicounty litigation (MCL) in New Jersey.
Additionally, dozens of NAION lawsuits filed in federal court were consolidated into multidistrict litigation (MDL) in December 2025. Grouping cases together allows them to move through the legal process in a coordinated fashion. That can lead to faster and more uniform results for people who have filed cases.
-
February 5, 2026
More lawsuits involving drugs like Saxenda have now been filed in federal court. Over the last few weeks, more than 100 new cases have been filed in federal court by people who say that they experienced serious stomach issues or blindness after taking a GLP-1.
-
January 22, 2026
Many lawsuits involving Saxenda and other GLP-1 drugs are now active in federal court. There are currently more than 3,000 active lawsuits involving stomach issues like gastroparesis. There are also 29 federal cases involving vision loss, but that number is expected to grow significantly in the coming months as more lawsuits are filed.
-
December 15, 2025
After the creation of an MCL for state cases last month, a federal multidistrict litigation (MDL) has now been approved for GLP-1 vision loss lawsuits. This will allow dozens of cases filed across federal court to be brought together before one judge for coordinated proceedings.
-
November 20, 2025
The New Jersey Supreme Court has approved the formation of a multicounty litigation (MCL) for vision loss lawsuits involving drugs like Saxenda. This is a big step for these lawsuits that should lead to more efficiency for cases filed in state court.
-
July 19, 2025
Plaintiffs’ attorneys in multidistrict litigation (MDL) 3094, an MDL that covers gastrointestinal injuries linked to GLP-1 drugs, filed a motion to create a vision injury track within MDL 3094. Currently, there are about 140 cases in the MDL for vision loss and total blindness.
-
June 12, 2025
Plaintiffs’ lawyers filed a petition to create a multicounty litigation in New Jersey for NAION lawsuits involving Ozempic and Wegovy. Saxenda users may be eligible to join this litigation.
-
June 6, 2025
A Saxenda and Wegovy user filed a lawsuit claiming her use of the drugs caused NAION and that manufacturer Novo Nordisk didn’t warn users of the potential risk.
Saxenda Lawsuits for Gastrointestinal Problems
In addition to NAION lawsuits, people have filed Saxenda lawsuits because of gastrointestinal problems like paralyzed stomach (gastroparesis) and bowel obstruction.
The gastroparesis litigation also includes Ozempic and Mounjaro lawsuits.
Other GLP-1 Vision-Loss Lawsuits
There are 21 lawsuits in New Jersey alleging Novo Nordisk didn’t warn that their GLP-1 diabetes and weight loss drugs Ozempic and Wegovy can trigger NAION.
In June 2025, plaintiffs’ attorneys asked New Jersey’s Supreme Court to consolidate NAION cases in an MCL. Comments or objections to the MCL are due by August 29, 2025.
This litigation has just started. There have been no trials or settlements.
Am I Eligible to File a Saxenda Lawsuit?
You might be eligible to file a Saxenda NAION lawsuit if you took the drug and were diagnosed with NAION afterward.
If you aren’t sure whether you qualify to file a Saxenda lawsuit, contact a lawyer. They can tell you if you qualify.
When you talk to an attorney, make sure to gather your medical records, receipts, vision test results and other evidence that shows you took Saxenda and later developed vision loss.
What Is the Average Saxenda Settlement Payout?
Some lawyers estimate the average settlement payout for eye injuries like those associated with Saxenda, including vision loss or total blindness, could be $500,000 or more.
Since there haven’t been any settlements or jury verdicts for Saxenda eye injuries, the exact settlement payout can’t be determined yet. No exact amount is guaranteed.
What Factors Affect Saxenda Settlement Amounts?
Factors that affect Saxenda settlement amounts include how serious your injury is and what other physical, mental or emotional losses you experienced because of your use of the drug.
- Age and occupation
- Your age and what you did for a living may impact your payout. For example, if you lost your job and stand to lose substantial future earnings, that will likely affect your claim.
- Medical expenses
- Your settlement may cover your past, present and future medical bills.
- Pain and suffering
- Having a permanent disability, psychological distress or loss of quality of life can affect your payout.
- Severity of vision loss
- The extent of your vision loss can significantly impact your settlement. For example, if you completely lost vision in both eyes, you may receive more than if you lost vision in one eye.
- What state you live in
- Where you live may limit the amount a jury can award you.
How to File a Saxenda NAION Lawsuit
The easiest way to file a Saxenda NAION lawsuit is to sign up for a free case review through Drugwatch. You can learn about your legal rights and whether you have a case, with no pressure to file a lawsuit.
Plus, you don’t pay anything to your lawyer unless they win your case. There’s no risk involved.
- Get a free, no-obligation attorney consultation.
- Sign a contingency-fee retainer and hire your attorney.
- Gather prescription and medical records from your primary doctor and ophthalmologist.
- Your lawyer files your lawsuit before the time limit expires.
- Plaintiffs and defendants exchange information, and experts provide evidence that Saxenda may increase the risk of NAION.
- Your lawyer negotiates a settlement or prepares your case for trial.
When you hire your lawyer, they can answer your questions about what to expect. They will also coach you every step of the way.
What Is Saxenda & How Does It Work?
Saxenda is a prescription medication that’s approved by the U.S. Food & Drug Administration (FDA) to help people with obesity or who are overweight lose weight. It’s also approved for children aged 12 and up who meet specific criteria.
The active ingredient is liraglutide, which mimics the action of a natural hormone in the body called GLP-1. This hormone helps control blood sugar in patients with Type 2 diabetes. Saxenda contains the same active ingredient as Victoza, an FDA-approved drug that treats Type 2 diabetes.
People who take Saxenda lose weight because the drug decreases hunger, increases feelings of fullness and slows the movement of food out of their stomach. Studies show that users typically lose 3% to 8% of their initial body weight. Some people may lose more.
Ozempic and Wegovy are similar medications, but they contain another GLP-1 drug called semaglutide. Ozempic is FDA-approved to treat Type 2 diabetes, while Wegovy is approved for weight loss.
Saxenda Side Effects & Safety Warnings
Saxenda side effects include mild, common side effects like constipation, diarrhea and nausea. Serious side effects include kidney problems from dehydration, severe stomach issues and thyroid tumors.
| Common Side Effects | Serious Side Effects |
|---|---|
| Constipation | Dehydration that can cause kidney problems |
| Diarrhea | Depression or suicidal thoughts |
| Dizziness | Food or liquid getting into lungs during surgery |
| Headache | Gallbladder problems |
| Injection site reaction | Higher risk of low blood sugar |
| Low blood sugar | Increased heart rate |
| Nausea | Pancreatitis (inflammation of the pancreas) |
| Stomach pain | Serious allergic reactions |
| Tiredness | Severe stomach problems |
| Upset stomach | Thyroid tumors |
| Vomiting |
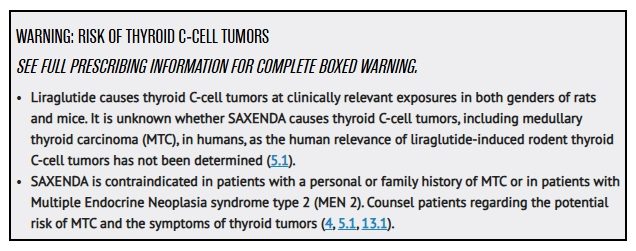
The FDA added a black box warning to Saxenda due to a potentially increased risk of thyroid C-cell tumors. The warning states that researchers found these tumors in animal studies for liraglutide, but they don’t know if these tumors can form in humans.
Scientific Evidence Linking Saxenda to NAION
Studies have linked Saxenda to NAION. A 2024 study in Epic Research found patients who took liraglutide had a 179% greater likelihood of being diagnosed with NAION than those prescribed other diabetes medications.
A 2025 research letter published in JAMA Ophthalmology found liraglutide increased the risk of NAION by 25%. It also found that semaglutide increased the risk of NAION by 39%.
FAQs About Saxenda NAION Claims
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.



