Topamax
Topamax (topiramate) is FDA-approved to treat seizures in adults and children and reduce the frequency of migraine headaches in people 12 years of age and older. While Topamax is effective, it has potentially serious side effects like eye issues, kidney stones and metabolic acidosis (acid buildup).
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Michael Gabay, Pharm.D., JD, BCPS, FCCP
- Last update: December 1, 2025
- Est. Read Time: 6 min read
Topamax is a prescription medication known as an anticonvulsant. The U.S. Food and Drug Administration (FDA) first approved Topamax for the treatment of seizures in 1996. The regulatory agency expanded the drug’s uses to include migraine prevention in 2004.
By 2007, Topamax controlled 21% of the $10.2 billion epilepsy drug market, according to Bloomberg News. Topamax sales reached $2.7 billion in 2008.
As people used Topamax more widely in the 2000s, users began to report various side effects. As a result, the FDA added several warnings to the drug’s label for suicidal thoughts and behaviors, eye disorders that could lead to permanent vision loss, decreased sweating with an elevated body temperature and increased risks for high levels of acid in the blood.
In 2011, the FDA warned of Topamax’s link to birth defects (cleft lip and cleft palate), and injured users began filing Topamax lawsuits. Also in 2011, J&J recalled roughly 57,000 bottles of Topamax because of possible chemical contamination.
Topamax lost its patent protection in 2009. Today, generic topiramate is widely available in the U.S.

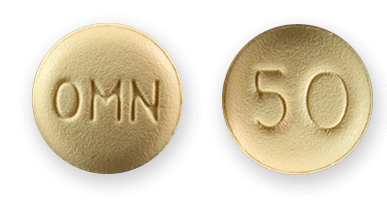
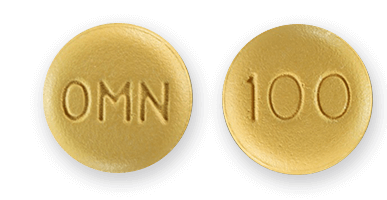
FDA-Approved Topamax Uses
When Topamax first hit the market, the FDA limited the drug for use in treating seizures from various forms of epilepsy. After about eight years, the agency added migraine prevention as an FDA-approved use. Seizures and migraines continue to be the only government-approved uses for Topamax in the U.S.
Seizures
During a seizure, brain cells work much faster than they normally would. Topamax stops seizures when they first begin by preventing brain cells from working in overdrive.
Topamax gained FDA approval in 1996 for the treatment of various forms of epilepsy, a neurological disorder characterized by recurrent, uncontrolled seizures. Doctors also prescribe the medicine for patients two years or older whose seizures are associated with Lennox-Gastaut syndrome, a disorder that accounts for up to 10% of all cases of childhood epilepsy. Topamax is not approved for use in children younger than two years of age.
- Partial onset seizures
- Primary generalized tonic-clonic seizures
- Seizures associated with Lennox-Gastaut syndrome
Migraines
In 2004, the FDA approved Topamax for the prevention of migraine headaches in people 12 years of age and older. Topamax does not relieve the pain of a migraine after it starts, but experts believe that it reduces migraine frequency by calming overactive pain-signal firing in nerve cells.
Topamax’s manufacturers recommend doctors “start low and go slow” when prescribing Topamax. This means a doctor will likely prescribe a low dose to start and then slowly increase the amount of Topamax a patient takes until reaching the dosage that works best for the patient.
Off-Label Use for Weight Loss, Psychiatric Disorders
Some people also use Topamax to control binging and purging, and to promote weight loss. However, this is not an FDA-approved use. Although it is illegal for drug makers to promote drugs for off-label uses, doctors can legally prescribe any drug they consider appropriate to treat a patient’s condition.
Topiramate, the active ingredient in Topamax, is also a main ingredient in the weight-loss drug Qsymia, along with phentermine. The FDA approved Qsymia in 2012. A year earlier, the FDA rejected the same drug — then called Qnexa — because of potential adverse effects, including psychiatric problems, cardiovascular issues and birth defects.
The FDA label warns Qsymia may cause mood and sleep disorders, cognitive impairment and increased heart rate. It could also lead to suicidal thoughts, and people who suddenly stop taking it can suffer seizures.
- Alcohol dependency
- Bipolar disorder
- Borderline personality disorder
- Cocaine and methamphetamine addiction
- Obsessive-compulsive disorder
- Post-traumatic stress disorder
Topamax Side Effects
Topamax has a number of common side effects, which its manufacturer states are “mild to moderate.” One common side effect of the drug is somnolence, or sleepiness. Some doctors and patients have dubbed the drug “Sleepomax” because of its heavy sedative effects.
- Abdominal pain
- Abnormal vision
- Anorexia
- Burning or prickling in the hands, arms, legs and feet (paresthesia)
- Diarrhea
- Difficulty with memory
- Dizziness
- Fatigue/sleepiness
- Fever
- Nausea
- Nervousness
- Numbness (hypoesthesia)
- Slowing down of thought and movements (psychomotor slowing)
- Speech problems
- Taste change
- Upper respiratory tract infection
- Weight loss
Serious Side Effects
Topamax may also cause rare but serious side effects such as eye complications, metabolic acidosis and kidney stones. Metabolic acidosis is a buildup of acid in the body that can lead to issues such as kidney damage, coma or death if left untreated.
According to its prescribing information, Topamax can cause significant cognitive side effects, including depression and mood disorders.
Additionally, Topamax can affect your vision and slow your motor skills. This may interfere with your ability to operate a vehicle or heavy machinery.
Some patients also reported suicidal thoughts and behavior when taking the drug. The FDA requires the packaging of most anti-seizure medications, including Topamax, to contain a warning about the possibility of suicidal thoughts.
- Acute nearsightedness
- Cognitive dysfunction
- Glaucoma from increased eye pressure
- Suicidal behavior
If you notice any symptoms of these conditions, you should report them to your health care provider immediately.
If necessary, your provider may help you develop a plan for coming off the medication gradually. Because stopping Topamax suddenly can cause seizures, even in people without epilepsy, you should not stop taking the medication without consulting your provider first.
Most Recent Side Effects Information
As of November 2025, the FDA Adverse Events Reporting System (FAERS) had over 14,000 reports of adverse reactions among patients taking Topamax. Seizures, headaches, weight loss and nausea were among the most frequently reported side effects. Additionally, 1,444 reports claimed the drug was ineffective.
| FDA Adverse Event Reports for Topamax Side Effects | Cases |
|---|---|
| Total Cases Reported | 14,275 |
| Serious Cases (Including Deaths) | 10,763 |
| Deaths | 600 |
Around 75% of reported side effects listed in the FAERS database involve serious reactions. However, the data does not establish a definitive relationship between Topamax and the listed side effects.
Many people take Topamax safely with no adverse reactions. However, you should be aware of potential side effects before taking any medication, including Topamax.
- Prickling/Tingling Sensation (40%)
- Weight Decrease (17%)
- Drowsiness (15%)
Upper respiratory infection was the top side effect in children taking Topamax, followed by weight loss.
FDA Warnings
Over the years, post-marketing studies have revealed several serious health risks associated with Topamax. In response, the FDA has continually mandated that all topiramate labels and medication guides contain updated warnings and precautions associated with its use.
-
2004
The FDA required a warning about metabolic acidosis (an increase in the level of acid in the blood), oligohydrosis (decreased sweating) and hyperthermia (increased body temperature).
-
2006
The FDA mandated that J&J add a warning about serious eye disorders, such as acute myopia and secondary angle-closure glaucoma, which can lead to permanent visual loss.
-
2008
Per the FDA’s guidance, J&J added a warning about an increased risk of suicidal thoughts and behaviors.
-
2011
The FDA required a warning about the risks of oral birth defects, including cleft lip and cleft palate. The FDA reinforced that Topamax can cause fetal harm when taken during pregnancy.
Topamax’s label also includes warnings regarding depression and mood problems, kidney stones and the need to withdraw the drug gradually to reduce the potential for increased seizures.
Topamax Drug Interactions
Topamax interacts with other substances, and patients should be aware of these interactions. The body digests Topamax in the liver and eliminates it from the body through the kidneys. Taking other medicines that are digested in the liver at the same time as Topamax may affect how quickly the drug works and leaves the body. People with liver disease and those with a history of kidney stones must be cautious about taking the drug.
The body gets rid of Topamax faster if taken at the same time as certain seizure medicines, including carbamazepine (Tegretol, Tegretol XR, Carbatrol), phenytoin (Dilantin, Phenytek), phenobarbital and primidone. Taking Topamax with valproic acid (Depakene) or a similar divalproex sodium drug (Depakote) has links to hypothermia and hyperammonemia.
Topamax makes birth control pills less effective, so the chances of becoming pregnant while on Topamax are greater. If a patient takes Topamax while pregnant, their baby has a higher risk of oral birth defects such as cleft lip and cleft palate.
People should not drink alcohol or take sedatives or tranquilizers, such as Valium and Xanax, while taking Topamax. Topamax suppresses the activity of the enzyme carbonic anhydrase in the body. Using the drug with any other carbonic anhydrase inhibitor, like zonisamide or acetazolamide, may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation. Topamax may also interact with lithium, resulting in increased lithium levels.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




