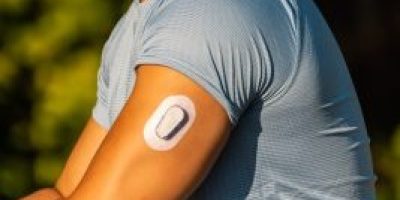
Dexcom G6 Glucose Monitors Lawsuit
Dexcom G6 continuous glucose monitoring (CGM) lawsuits claim the system failed to give accurate readings or critical alerts, leading to severe injuries and death. Lawsuits allege the company failed to warn about risks and produced a defective product.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Last update: February 3, 2026
- Est. Read Time: 8 min read
Why Are People Filing Dexcom G6 Lawsuits?
People are filing Dexcom G6 lawsuits claiming that defects and inaccurate readings led to serious injuries. These include hypoglycemia, hyperglycemia, severe skin reactions and death.
One U.S. Food & Drug Administration device report complained that the G6 sensor failed at least 25% to 33% of the time.
Dexcom G6 Glucose Monitor Lawsuits Filed for Problems and Malfunctions
Lawsuits claim that the Dexcom G6 continuous glucose monitor (CGM) is defectively designed. This can lead to malfunctions and inaccuracies that may fail to alert people when their blood sugar levels are too low or too high.
For example, Katelyn Dickson filed a lawsuit when her Dexcom G6 didn’t warn her that her blood sugar had dropped to dangerous levels. She crashed her car at 65 mph into a concrete driveway and culvert. Dickson was pregnant and had her young infant in the car with her.
In a wrongful death lawsuit filed by Joseph Higginbottom on behalf of Anthony Higginbottom, Anthony’s G6 inaccurately read his glucose levels as extremely high. This caused too much insulin to be administered, leading to a rapid decrease in blood glucose.
His G6 failed to warn him. He passed out from hypoglycemia while driving and crashed into another vehicle. His injuries were fatal.
What the Dexcom G6 Lawsuit Allegations Say About the Device
Lawsuits allege several counts of misconduct against Dexcom, including failure to warn the public about the risks of the device and manufacturing a defective product.
- Defective design:
- Lawsuits claim that the G6 is defective and prone to malfunctions as well as reading inaccuracies that can cause serious harm or put people’s lives at risk.
- Failure to warn:
- Dexcom didn’t properly warn the public about the risk of hypoglycemic/hyperglycemic events, loss of consciousness, coma, seizure, death and other injuries linked to using the Dexcom G6.
- Negligence:
- The defendant failed to properly test, design and market the G6.
All of these problems led plaintiffs to suffer serious injuries and death, according to the lawsuits.
Dexcom G7 Involved in Wrongful Death Lawsuit
In addition to G6 lawsuits, people have also started filing G7 lawsuits claiming the device failed to alert them to dangerous glucose levels.
Jennifer Wisdom-Schepers filed a wrongful death lawsuit on behalf of Michael Schepers. Michael successfully managed his blood sugar until he switched to Dexcom G7 with an insulin pump.
One day, he suffered some gastrointestinal symptoms, but his G7 read normal blood sugar levels. The next day, he suffered a heart attack. The hospital found his blood glucose was at 1,651mg/dL, a dangerous and potentially fatal level.
He suffered from diabetic ketoacidosis, elevated potassium that caused his heart attack, kidney failure and lactic acidosis. He never regained consciousness and died the following day.
Dexcom G6 and G7 Class Action Lawsuit
In addition to G6 and G7 individual injury lawsuits, a group of people filed a class action lawsuit against Dexcom.
Class action plaintiffs allege that Dexcom marketed its products as effective for monitoring blood glucose levels and for warning people when levels were too high or low. The lawsuit claims that Dexcom misrepresented these claims and that they are false.
Plaintiffs say Dexcom violated consumer protection laws. They want refunds for their purchases.
Dexcom Faces Investor Lawsuit
In addition to individual plaintiffs seeking refunds, Dexcom G6 and G7 investor security class action lawsuits have also been filed.
These lawsuits are for investors who purchased Dexcom securities between January 8, 2024, and September 17, 2025. The lawsuit alleges that Dexcom made unauthorized design changes and that these modifications made the products less reliable.
Who Qualifies To File a Dexcom G6 Lawsuit?
You may qualify to file a Dexcom G6 lawsuit if you or a loved one used a G6 CGM and it didn’t work properly, leading to severe injuries.
- Documented the malfunction of the Dexcom G6 device, such as an app crash, missed alerts or other problems with the device.
- Have documented G6 use before your injury.
- Have lost wages, medical expenses or emotional damages because of injuries from a Dexcom G6 continuous glucose monitor.
- Required hospitalization or emergency care because of incorrect readings from a Dexcom G6 CGM.
- Suffered hypoglycemia, hyperglycemia or other serious injuries because of Dexcom G6 malfunctions.
If you lost a loved one because of Dexcom G6 complications, you can file a wrongful death claim on their behalf.
Dexcom G6 Recalls, FDA Warnings and Safety Notices
The FDA announced two recalls in 2025 for the Dexcom G6. One was for Android app problems and a second was for defective speakers in the G6 receivers.
The agency also sent a warning letter to Dexcom after it found the company made changes to the devices without submitting a premarket notification to the FDA.
Dexcom G6 Recalls for Receiver or App Problems
In October 2025, the FDA posted a Dexcom G6 recall because the G6 Android app can suddenly stop working. This could cause users to not receive alarms, alerts, notifications or estimated glucose values.
Previously, in June 2025, the FDA posted a Dexcom G6 receiver recall because a defective foam or assembly error can lead to missed audible alerts for high or low blood glucose values.
Both of these recalls were classified as Class I recalls, the most extreme type that may lead to serious injury or death. In this case, potential injuries include severe hyperglycemia, diabetic ketoacidosis and hyperosmolar hyperglycemic state.
Untreated hypoglycemia or hyperglycemia may lead to vomiting, seizures, loss of consciousness or death.
FDA Sends Warning Letter to Dexcom
In a March 2025 warning letter to Dexcom, the FDA noted several issues from an inspection of Dexcom’s California offices. One of the most important findings was that Dexcom did not properly evaluate and validate a design change for the resistance layer of G6 and G7 sensors.
The manufacturer also didn’t meet its primary endpoint of proving the new and old designs were equivalent, with one seemingly performing worse than the other when it comes to accuracy.
How Recalls and FDA Actions Support Dexcom G6 Lawsuit Allegations
Recalls and FDA warnings support Dexcom G6 allegations by demonstrating possible inconsistencies or defects in the design and manufacture of the Dexcom G6.
These recalls also clearly list the potentially serious injuries associated with faulty alerts and readings that are at the center of lawsuits.
Dexcom G6 Lawsuit Updates and News
Many of the Dexcom lawsuits are in the early stages. There aren’t any public settlements or trials scheduled.
What Happened with Dexcom G6 Lawsuits in 2025?
By late 2025, multiple law firms report ongoing Dexcom G6 injury investigations and active case filings in state and federal courts. The most recent update in the litigation is the filing of Dexcom G6 and G7 class action complaints.
The first class action filed in September 2025 is for refunds of the purchase price of the CGMs. The second filed in November 2025 is an investor class action complaint.
The lead plaintiff deadline in the investor class action complaint is Dec. 26, 2025.
What Could Happen Next in Dexcom G6 Litigation?
For the class action lawsuit to proceed, courts must determine if it meets the requirements for certification. Then, the class members either opt into or out of the lawsuit before a settlement is negotiated.
If a settlement can’t be negotiated, the case may proceed to trial.
In the event the class action lawsuit is not certified, federal courts may consider consolidating certain Dexcom G6 cases into a multidistrict litigation (MDL). MDLs help streamline the legal process when there are multiple similar cases.
If an MDL is not created, cases will proceed individually in their respective courts.
How To File a Dexcom G6 Lawsuit
If you are considering filing a Dexcom G6 lawsuit, the first step you can take is to get a free case review with Drugwatch. It’s easy to sign up using the forms on this page. Then, we can match you with an experienced medical device mass torts attorney.
Steps To Take if You Had Dexcom G6 Glucose Monitor Problems
If you experienced severe hypoglycemia, hyperglycemia or another major event while using a Dexcom G6, consider taking the following steps:
- Seek immediate medical care and follow your provider’s advice for stabilizing your glucose and treating complications.
- Preserve your Dexcom devices (sensor, receiver and transmitter) and, if possible, do not discard them until you speak with a lawyer.
- Save or download app data, screenshots and any communications from Dexcom or your pharmacy about recalls or device corrections.
These actions can help protect your health and strengthen your potential legal claims.
Medical Records, Device Data and Evidence Lawyers May Need
Attorneys evaluating a Dexcom G6 case may request:
- Device and app data showing glucose trends, alerts (or lack of alerts) and error messages before and during the incident.
- Medical records documenting your diabetes history, the event (ER/ICU notes) and any diagnoses such as diabetic ketoacidosis, seizure or hypoglycemic encephalopathy.
- Proof of Dexcom G6 use (prescriptions, device serial numbers, receipts and photos) and details about app versions or receivers used at the time.
In some cases, lawyers may also review recall notices, FDA communications and expert analyses to connect your specific experience with known device defects.
What To Expect When You Contact Drugwatch for a Free Case Review
When you contact Drugwatch, you typically complete a brief form or call the number at the top of the page to speak with a representative from one of our vetted partner firms.
You’ll receive a free, no‑obligation evaluation of your potential Dexcom G6 claim from our partners. They will ask you about your Dexcom G6 use, your injuries and any hospitalizations.
If they tell you that you qualify, you can decide whether you want to pursue a claim.
Dexcom G6 Settlement and Compensation
Since there haven’t been any settlements or trials, we can’t accurately estimate the exact amount of a Dexcom G6 settlement or compensation an individual may receive.
However, we know what types of compensation are typically included in product liability and mass tort injury cases.
Types of Compensation in Dexcom G6 Lawsuits
Compensation in Dexcom G6 lawsuits may include:
- In wrongful death cases for people who died because of their Dexcom G6 injuries, plaintiffs may claim funeral costs and loss of financial and emotional support to surviving family members.
- Lost wages or reduced earning capacity if injuries interfered with work.
- Pain and suffering, emotional distress and loss of enjoyment of life.
- Past and future medical expenses related to hypoglycemia, hyperglycemia, diabetic ketoacidosis, seizures or other complications.
Punitive damages may be available in some jurisdictions if a jury finds Dexcom’s conduct especially reckless or malicious.
Factors That May Affect Dexcom G6 Settlement Amounts
Potential settlement values can vary based on:
- Your age, earning history, medical needs and any pre‑existing conditions. This could include whether or not your diabetes was well-controlled prior to the injury.
- Severity and permanence of the injury (for example, temporary hospitalization vs. long‑term brain injury).
- Strength of evidence linking a specific G6 malfunction, such as a recalled receiver or affected app version, to the injury.
Regulatory findings, internal company documents obtained in discovery and patterns of similar injuries may also influence settlement negotiations.
Dexcom G6 Lawsuit FAQs
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.