GranuFlo & NaturaLyte: Side Effects, Recalls & Lawsuit Information
GranuFlo and NaturaLyte are dialysis concentrates manufactured by Fresenius. Internal 2011 documents showed Fresenius knew of a six times higher cardiac arrest risk with the products, but did not alert the public until a 2012 FDA recall. Some patients who were adversely affected filed lawsuits.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Kenneth S. Fill, Pharm.D., MBA
- Last update: November 24, 2025
- Est. Read Time: 8 min read
- What Are GranuFlo and NaturaLyte?
- Common GranuFlo and NaturaLyte Side Effects
- Serious GranuFlo and NaturaLyte Side Effects
- FDA Warnings and Recalls
- Why Were GranuFlo Lawsuits Filed?
- Alternatives and Patient Safety Considerations
- GranuFlo and NaturaLyte in the News
- Legal Options After GranuFlo Injuries
What Are GranuFlo and NaturaLyte?
GranuFlo and NaturaLyte are acid concentrates used in dialysis treatments. Both products are made by Fresenius Medical Care and distributed to dialysis centers across the United States. GranuFlo is a powdered concentrate, while NaturaLyte is the liquid counterpart.
Fresenius marketed both products as safe and effective. However, they were later linked to increased risk of heart attack and other major cardiac events.
Common GranuFlo and NaturaLyte Side Effects
Many side effects associated with GranuFlo and NaturaLyte are mild and typical of dialysis treatments. These symptoms include nausea, itching and muscle cramps.
There are also more significant side effects associated with dialysis treatments, including GranuFlo and NaturaLyte. These include:
- Anemia
- Irregular heartbeat
- Low blood oxygen
- Sleep problems
If you experience any of these side effects, alert your doctor immediately.
Serious GranuFlo and NaturaLyte Side Effects
There are many serious side effects linked to NaturaLyte and GranuFlo. These include cardiac death, heart attacks, stroke and more.
According to a 2011 Fresenius memo, 941 patients suffered cardiac arrest due to these products. The company’s analysis also showed that dialysis patients using GranuFlo or NaturaLyte were roughly six to eight times more likely to experience fatal cardiac arrest than patients using other dialysis concentrates.
Metabolic Alkalosis and Low Potassium Levels
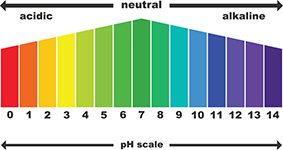
The sodium diacetate in GranuFlo and NaturaLyte can lead to a rapid increase in your blood bicarbonate levels. While bicarbonate is a natural chemical in your blood, too much may make your blood more alkaline than it should be. This can cause metabolic alkalosis, especially if the products are not given properly.
Metabolic alkalosis is a significant risk for dialysis patients using GranuFlo and NaturaLyte. It can cause severe and life-threatening complications, including an increased risk of heart attacks, cardiopulmonary arrest, cardiac death and stroke. Some patients even died within 48 hours of treatment.
“My major concerns with NaturaLyte and GranuFlo include risks of metabolic alkalosis as well as associated cardiovascular complications,” Dr. Michael McKinney, with medical weight loss center Healthy Outlook, told Drugwatch.
“I pay close attention to electrolyte concentrations and blood pressure levels in patients on dialysis to minimize cases such as hypotension and imbalances in electrolytes.”
Researchers writing in Seminars in Dialysis studied hemodialysis patients over ten years. They found that older patients with high bicarbonate levels had more heart issues and lower blood pressure during dialysis. These patients were more likely to have persistent alkalosis, which could lead to serious heart problems.
- Confusion
- Hand tremors
- Light-headedness
- Muscle twitching
- Nausea
- Prolonged muscle spasms
- Vomiting
Low potassium levels (hypokalemia) often accompany metabolic alkalosis in dialysis patients. An internal memo from Fresenius noted that patients with low potassium and high bicarbonate levels had an even greater risk of cardiopulmonary arrest.
Hypokalemia can cause muscle weakness, low blood pressure and irregular heartbeats. When combined with high bicarbonate levels, your risk of severe cardiac events increases dramatically.
“[Health care] providers should enforce strict safety protocols, including continuous lab testing and individualized treatment modifications when dialyzing with GranuFlo and NaturaLyte,” McKinney said.
Heart Attacks
GranuFlo and NaturaLyte have been associated with a higher risk of heart attacks. In 2011, a study conducted by their manufacturer found that improper use of GranuFlo could increase the likelihood of heart attacks in dialysis patients.
The reason for this is that incorrectly mixed GranuFlo solutions can lead to metabolic alkalosis, increasing your risk of heart attacks
Case Study: Sudden Cardiac Arrest During Dialysis
In November 2011, Stephanie Boone from Texas was being treated for her serious kidney condition. She was receiving dialysis, a treatment that helps filter waste from the blood when the kidneys can’t do it on their own.
Boone got therapy at the Fresenius Medical Care Southeast Kidney Center. While there, she received either GranFlo or NaturaLyte.
Dangerous Side Effects
During her dialysis treatment, Boone suffered cardiac arrest. Medical personnel immediately transported her to a nearby hospital. According to court documents, she claimed the cardiac arrest was directly linked to the dangerous effects of GranFlo or NaturaLyte.
GranuFlo contains acetic acid and sodium diacetate, which the liver converts into bicarbonate. This process can dangerously increase bicarbonate levels beyond those prescribed by a physician.
Elevated bicarbonate levels can lead to metabolic alkalosis. With this condition, the blood becomes too alkaline, increasing the risk of severe complications such as heart attacks.
Impact and Consequences
Stephanie Boone’s cardiac arrest caused significant medical and emotional distress. She endured severe physical trauma and faced potential long-term health impacts as a result of the defective dialysate solutions.
Cardiopulmonary Arrest
Cardiopulmonary arrest is another severe side effect linked to GranuFlo and NaturaLyte. With this condition, your heart and lungs suddenly stop functioning.
Data from Fresenius’ internal memo indicated that patients with high bicarbonate levels before their dialysis faced a roughly six to eight times greater risk of cardiopulmonary arrest and sudden cardiac death.
The memo recommended closely monitoring patients with high bicarbonate levels, defined as 24 mEq/L or higher, and adjusting treatment to keep the levels within a safer range. Doctors should regularly check and adjust bicarbonate levels in these patients.
Stroke
Strokes are a serious risk associated with the use of GranuFlo and NaturaLyte. A stroke happens when the blood supply to part of your brain is interrupted or reduced. This prevents your brain tissue from receiving oxygen and nutrients, which can lead to brain damage or death.
The potential for elevated bicarbonate levels when using GranuFlo and NaturaLyte can lead to a chemical imbalance in your blood, increasing your risk of strokes. Metabolic alkalosis can exacerbate pre-existing conditions that may lead to stroke.
Additionally, the American Heart Association reports that low potassium levels can increase your risk of stroke. This is a concern for patients receiving GranuFlo and NaturaLyte.
Other Side Serious Effects of GranuFlo and NaturaLyte
Metabolic alkalosis and hypokalemia are also associated with other dangerous side effects for GranuFlo and NaturaLyte patients. These can include:
- Cardiac arrhythmia: This irregular heartbeat can cause sudden cardiac arrest.
- Low blood pressure (hypotension): An imbalance in your blood chemistry can result in hypotension, which can cause dizziness, fainting and other complications.
- Low oxygen levels (hypoxemia): Reduced oxygen in your blood can cause serious complications, including organ damage.
If you or a loved one has suffered from side effects related to GranuFlo or NaturaLyte, seek medical advice.
FDA Warnings and Recalls
In the wake of the 2011 Fresenius memo and growing concerns about cardiac events linked to GranuFlo and NaturaLyte products, the U.S. Food & Drug Administration (FDA) took regulatory action in 2012. This included a recall, label changes and safety alerts.
FDA Class I Recall (2012)
The FDA issued a Class I recall for GranuFlo and NaturaLyte on March 29, 2012. This is the most severe class of FDA recall, indicating that “there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.”
This recall was prompted by the release of the internal documents from Fresenius. The listed reason for the recall was the risk of alkalosis.
Labeling Changes and Safety Alerts
The FDA’s recall action was triggered when Fresenius’s internal memo warning of cardiac risks was leaked to federal regulators. Fresenius only distributed a condensed two-page version of their safety warnings to physicians outside their own network after this leak.
This warning was distributed on the same day as the recall, March 29, 2012.
Why Were GranuFlo Lawsuits Filed?
The risks associated with GranuFlo and NaturaLyte led to injuries and deaths. Consequently, plaintiffs filed GranuFlo lawsuits alleging that the manufacturer failed to adequately warn about the potential risks and that the product was dangerous.
- Failure to warn:
- The November 2011 memo shows Fresenius knew their products had a six to eight times higher risk of cardiopulmonary arrest and sudden cardiac death, yet they continued marketing GranuFlo and NaturaLyte without warning outside physicians or patients for months.
- Product liability:
- Plaintiffs alleged that the acetate in these product formulas produced higher bicarbonate conversion than other dialysis concentrates, so it was designed dangerously. They also claimed that product marketing downplayed the risks of GranuFlo and NaturaLyte compared to other dialysis concentrates.
As both the manufacturer and distributor of these concentrates, Fresenius was faced with negligence and wrongful death claims based on the claims outlined above.
Most lawsuits were resolved after Fresenius reached a $250 million settlement in 2016 with plaintiffs who claimed they, or their loved ones, were harmed by GranuFlo or Naturalyte within 48 hours of treatment.
The payout for each plaintiff depended on the severity of their injury and the supporting documentation to bolster their claim. Fresenius did not admit any wrongdoing and stood by its dialysis concentrate products.
Prior to the settlement, due to the large number of GranuFlo lawsuits, a federal MDL (multidistrict litigation) was formed in Massachusetts. An MDL consolidates multiple lawsuits that share a defendant, streamlining the litigation process and centralizing proceedings.
Alternatives and Patient Safety Considerations
Since the recall, dialysis centers across the U.S. have shifted to safer formulations of dialysis concentrates and improved monitoring protocols to prevent bicarbonate overload. Fresenius also revised their labeling to highlight the risks that were once downplayed.
For patients, the most important safeguard is open communication with their doctors. Asking about the type of dialysis concentrate being used and discussing any unusual symptoms, including chest pain, irregular heartbeat or sudden fatigue, can help reduce risks.
Finally, ongoing FDA oversight and mandatory safety alerts have strengthened dialysis safety standards, ensuring that dialysis products face closer scrutiny before reaching patients.
GranuFlo and NaturaLyte in the News
The risks linked to GranuFlo and NaturaLyte became national news after investigative reports revealed Fresenius’s memo warning doctors at its own clinics of cardiac dangers before patients or outside providers were informed.
Coverage from media outlets highlighted that many patients and families were blindsided by the recall, sparking public concern about transparency in the dialysis industry. The most recent GranuFlo headlines were for the settlements and verdicts that have come down since the recall.
For example, DaVita, a company that runs dialysis treatment centers, was ordered to pay over $383 million to three families of patients who died after being given GranuFlo in 2018. However, this was a malpractice lawsuit against DaVita, not against Fresenius.
Legal Options After GranuFlo Injuries
If you or a loved one experienced severe health issues after using GranuFlo or NaturaLyte, it may be possible to pursue compensation for medical bills, lost wages and more.
The plaintiffs involved in lawsuits typically qualified if they, or their loved one, experienced:
- Heart attack, cardiac arrest or sudden death within 48 hours of dialysis using GranuFlo or NaturaLyte.
- Metabolic alkalosis diagnosed shortly after treatment.
- Wrongful death connected to dialysis treatment.
You can discuss your case with an attorney to understand what legal options you may have.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.