Injectafer
Injectafer is a prescription intravenous iron replacement medication for adults with iron deficiency anemia who don’t do well with oral iron replacements. Common side effects include nausea, high blood pressure and flushing. It can also cause serious allergic reactions and hypophosphatemia.
- Medically reviewed by Kenneth S. Fill, Pharm.D., MBA
- Last update: May 19, 2025
What Is Injectafer?
The U.S. Food and Drug Administration originally approved Injectafer (ferric carboxymaltose injection) in 2013 to treat iron deficiency anemia (IDA). Unlike oral iron replacements, Injectafer is injected directly into the vein.
More than 1.7 million Americans have been treated with Injectafer, according to American Regent Inc., a subsidiary of drugmaker Daiichi Sankyo.
Providers may also prescribe Injectafer for certain cases of heart failure. In a 2022 clinical research trial, patients with heart failure with reduced ejection fraction taking ferric caroboxymaltose had improvements in both left and right ventricular function.
Most Injectafer side effects are mild or go away after a short time, but the drug has also been linked to hypophosphatemia, or abnormally low phosphate. Hypophosphatemia can cause symptoms including: Muscle weakness, heart failure, seizures, bone pain, coma and even death.
Some people who developed hypophosphatemia filed lawsuits against Injectafer’s makers for failing to warn them of the risk.
How Does Injectafer Work?
Injectafer comes in two strengths: 750 mg and 1,000 mg single dose vials. The usual dose is 1,500 mg given in two separate injections of up to 750 mg each at least seven days apart. In May 2021, FDA approved a 1,000 mg single dose option.
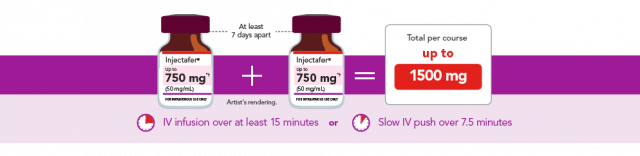
Injectafer works by slowly releasing iron into the body. An Injectafer infusion usually takes about 15 minutes. Health providers monitor the patient for at least 30 minutes after the infusion to monitor for allergic reactions.
Ferric carboxymaltose’s half-life is 7 to 12 hours. This means the drug can stay in the body for about 14 to 24 hours. Depending on a person’s body chemistry and health, this time may be more or less.
Health care providers may recommend repeating treatment if IDA reoccurs.
- Nausea (7.2%)
- Hypertension (4%)
- Flushing (4%)
- Injection site reactions (3%)
- Erythema (skin redness) (3%)
- Hypophosphatemia (2.1%)
- Dizziness (2.1%)
- Vomiting (2%)
- Injection site discoloration (1.4%)
- Headache (including migraines) (1.3%)
- Increased hepatic enzymes (1.2%)
- Dysgeusia (altered taste) (1.2%)
- Hypotension (1%)
- Rash (1%)
- Constipation (0.5%)
Precautions and Warnings
Injectafer isn’t for everyone. Make sure you talk to your doctor about all medical conditions you have, especially if you are on dialysis for kidney disease.
The drug’s label doesn’t list drug interaction, but make sure you tell your medical provider about all the medications and supplements you are taking.
Pregnancy
Data from clinical studies and postmarketing reports with Injectafer is insufficient to properly gauge the risk of miscarriage and birth defects in pregnant women. But iron infusions have been known to cause hypersensitivity reactions which can affect the fetus.
In animal studies, Injectafer caused fetal malformations and miscarriages.
Iron has been found in the breast milk of women treated with Injectafer.
Overdose
Overdosing on Injectafer can lead to too much iron in the body, a condition called hemosiderosis. Symptoms include fatigue, coughing, wheezing and difficulty breathing.
Hypophosphatemia
In February 2020, American Regent added a warning to the drug’s label for Symptomatic Hypophosphatemia.
“These cases have occurred mostly after repeated exposure to Injectafer in patients with no reported history of renal impairment,” and most cases resolve within three months, the drug’s prescribing said.
Patients who are prone to low phosphate levels should be monitored while taking Injectafer. Risk factors include gastrointestinal disorders, using medications that affect the kidneys, malnutrition and vitamin D deficiency.
Hypophosphatemia Diagnoses Lead to Litigation
Studies have found there are more cases of hypophosphatemia with Injectafer than other iron replacements.
Several patients filed Injectafer lawsuits against manufacturers claiming the companies knew about the risk but failed to adequately warn the public.
One plaintiff suffered shortness of breath, fatigue, bone pain and weakness for three years before doctors diagnosed her with hypophosphatemia after taking Injectafer.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.