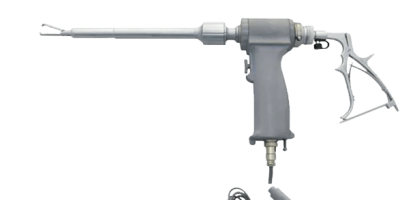
Morcellators: Risks, Cancer Concerns & Lawsuit Information
Power morcellators were once used in minimally invasive hysterectomies and fibroid removal surgeries. However, they have dangerous risks that have led to FDA warnings, recalls and lawsuits. Learn about side effects and patient safety concerns.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Dr. John A. Daller
- Last update: December 1, 2025
- Est. Read Time: 7 min read
What Are Morcellators?
Morcellator devices are surgical tools that break down large pieces of tissue so they can be removed through small incisions. They are often used in abdominal surgeries like hysterectomies (uterus removal) and myomectomies (uterine fibroid removal).
- Manual morcellators: Operated by hand
- Power morcellators: Use motorized blades for cutting tissue
The U.S. Food & Drug Administration (FDA) first cleared power morcellators for sale in 1991. The benefits of the devices included smaller incisions, less post-operative pain and faster recovery compared to open abdominal surgery.
However, hidden dangers of these devices came to light over time, including the device’s ability to spread undetected uterine cancer throughout the patient’s abdominal cavity.
Common Morcellator Side Effects
Surgery using a power morcellator can lead to typical surgical side effects, like bruising and infection. These devices can also cause the spread of fibroid tissue. This tissue is usually benign but can attach to other tissues and organs in your abdomen, causing more fibroids to grow.
- Bleeding
- Bowel obstruction
- Bruising
- Fibroid recurrence
- Infection
- Intra-abdominal abscesses
- Nausea and vomiting
- Pain and soreness
- Peritonitis (inflammation of the abdominal lining)
- The need for additional surgery
Some of these side effects are not unique to morcellators and can occur with any abdominal surgery.
Why Are Morcellators Considered Dangerous?
Power morcellators can spread undetected cancer cells during surgery. Uterine sarcomas are often undiagnosed before morcellator surgery. These aggressive cancers are hard to detect with standard imaging and are often mistaken for benign fibroids.
If you have undetected uterine cancer, the morcellator’s spinning blades can shred cancerous tissue and scatter malignant cells throughout your abdominal cavity. This can advance the disease from an early, treatable state to an aggressive, often fatal one.
One study about the dangers of morcellators found that 12 women had irregular tissue samples that were undiagnosed before surgery. Ten of these women had cancer cells. Types of cancer found included uterine myoma, endometrial stromal sarcoma and leiomyosarcoma.
A separate study found that women with undiagnosed uterine sarcoma who underwent power morcellation had a roughly 2.7 to 4.7 times higher risk of death than patients who had traditional surgery. In some cases, five-year survival rates dropped by over 37%.
How Common Are Uterine Sarcomas?
-
Uterine sarcomas are more common than previously thought. Over time, researchers and regulators have adjusted the risk estimates.
- Pre-2014: Experts estimated that roughly one in 10,000 women undergoing fibroid surgery had uterine sarcomas.
- 2014: The estimate was revised to reveal that one in 350 women may have undetected cancer during morcellation.
- Current Estimates: One in 225 to one in 580 women undergoing morcellation for hysterectomy or myomectomy have hidden uterine sarcoma.
Uterine Cancer After Morcellation
When cancer is diagnosed after morcellation, it is usually stage III or IV. These are advanced stages with a poor prognosis. A tumor is considered stage IV if it has spread to your lymph nodes, bladder, rectum, spine, lungs or other distant organs.
- Nausea or vomiting
- Pelvic or abdominal pain, mass or swelling
- Vaginal bleeding or spotting, between periods or after menopause
- Vaginal discharge
- Weight loss
If tumors affect other organs or bones like your spine, symptoms could include back pain or nerve issues.
Leiomyosarcoma and Morcellators
Leiomyosarcoma (LMS) is a rare cancer that is most commonly seen in smooth muscles of hollow organs like your uterus, stomach, bladder, blood vessels or intestines. Uterine LMS specifically is one of the more dangerous and aggressive types of uterine cancers.
Depending on when it’s caught, the five-year survival rate for uterine LMS is 13% to 61%. More favorable outcomes occur when a diagnosis is made early.
Power morcellators do not cause LMS, but they can lead to it spreading rapidly if it’s already present and undiagnosed. Before undergoing surgery, you should be evaluated to determine if you are at a higher risk of uterine cancer.
In a 2014 study, researchers evaluated how morcellation for hysterectomy patients can spread malignancies. They wrote, “patients considering morcellation should be adequately counseled about the prevalence of cancerous and precancerous conditions prior to undergoing the procedure.”
Data from the FDA supports this finding, indicating that LMS may be present in an estimated 1 in 495 to 1 in 1,100 women who undergo surgery for uterine fibroids.
Survival and Spread of Cancer
Typically, cancer is easiest to treat if it is localized, which means it has not spread. Treatment becomes more difficult if your cancer spreads to other organs (regionalized) or to the rest of your body (distant).
- Localized: Approximately six in 10
- Regionalized: Approximately three in 10
- Distant: Approximately one in 10
- Localized: Approximately seven in 10
- Regionalized: Approximately four in 10
- Distant: Approximately two in 10
- Localized: Almost 10 in 10
- Regionalized: Approximately nine in 10
- Distant: Approximately eight in 10
FDA Actions and Safety Warnings
The FDA first issued a warning in April 2014, highlighting the cancer risk and advising against routine use of morcellators in most women. However, according to a 2017 report from the U.S. Government Accountability Office, the FDA was aware of potential risks as early as 1991.
-
April 2014
The FDA issued its first warning about morcellator dangers, estimating that one in 350 women undergoing morcellation for hysterectomy or myomectomy may have undiagnosed uterine cancer.
-
April 2016
The FDA permitted the marketing of the first tissue containment system, PneumoLiner, for use with certain power morcellators to reduce the risk of spreading tissue during surgery. Manufacturers were required to warn patients that the PneumoLiner hadn’t been proven to reduce the risk of spreading cancer.
-
December 2017
The FDA reaffirmed its 2014 guidance, choosing not to ban morcellators but continuing to recommend strict caution and limited use in specific patient populations.
-
December 2020
The FDA issued product labeling guidance for laparoscopic power morcellators, requiring a boxed warning about the potential dangers of spreading undiagnosed cancer.
-
May 2023
The FDA released guidance with updated risk estimates and safety recommendations. Suggestions included avoiding power morcellators in women over 50 or those with suspected cancer, using tissue containment systems and informing patients about the full risks of spreading hidden cancer.
Morcellator Recalls and Market Impact
Following the FDA’s 2014 safety warning about the cancer risks linked to power morcellators, the medical community responded quickly. Hospitals began suspending use of the devices, and insurers re-evaluated coverage.
One study found that use of power morcellators dropped from 66.7% at the end of 2013 to 13.3% in the second quarter of 2018. A separate study found that there had been a decline in minimally invasive procedures since the “banning” of morcellators.
Johnson & Johnson’s Ethicon division, which produced various morcellator devices, voluntarily recalled the products. The company was the only manufacturer to recall devices based on the FDA’s safety warning.
In a letter to health care providers, Ethicon acknowledged the uncertainty around the devices’ safety.
“We believe Ethicon morcellation devices perform as intended and there are patients who can benefit from procedures using laparoscopic power morcellators, but the risk-benefit assessment associated with the use of these devices in hysterectomy and myomectomy procedures for removing fibroids remains uncertain,” the company stated.
Morcellator Lawsuits and Legal Claims
After developing advanced uterine cancer following fibroid surgery, many women and their families filed lawsuits against the makers of power morcellators.
Lawsuits alleged that defendants failed to adequately warn about the cancer risks associated with morcellators and didn’t properly test the devices before selling them. Plaintiffs also claim the defendants negligently designed and marketed the products.
Most of the legal action centered on Johnson & Johnson’s Ethicon division.
- Gyrus ACMI
- Karl Storz Endoscopy America
- LiNA Medical
- Olympus Corporation of the Americas
- Richard Wolf GmbH and Cook Medical
In 2015, the Judicial Panel on Multidistrict Litigation consolidated several morcellator cases against J&J’s Ethicon unit in the U.S. District Court for the District of Kansas. In 2016, roughly 100 power morcellator lawsuits were settled. Potential payouts ranged from $100,000 to $1 million.
Because of J&J’s settlements, both the company and plaintiffs’ attorneys asked a federal judge to dissolve a multidistrict litigation in which the lawsuits were consolidated. Only two cases were unresolved when the MDL was closed.
Alternatives to Morcellators
After the FDA issued warnings about the cancer risks linked to power morcellators, doctors began turning to safer surgical methods. Alternatives like vaginal or abdominal hysterectomies and abdominal myomectomy can help prevent the spread of hidden cancer.
- Abdominal hysterectomy and myomectomy: Surgeons use these techniques to remove your uterus and fibroids whole through an incision in your abdomen, minimizing the odds of cancer spread. The disadvantage to these procedures is a longer recovery time and a greater chance of infection and pain because of the larger incisions.
- Contained manual morcellation: This technique involves completing the surgery inside a bag that is inserted through a small incision in your abdomen. Surgeons use a manual morcellator to cut out fibroids or your uterus inside the bag and remove the specimen from your body through small incisions.
- Contained power morcellation: Surgeons insert a plastic bag into your abdominal cavity to enclose the space where surgery will take place. They then cut out fibroids or your uterus with a power morcellator within the bag to reduce the risk of spreading cancerous tissue.
- En bloc resection: In cases of cancer, doctors can cut out your entire tumor or organ as a whole to avoid spreading cancer. Since this is a major surgery, complications may be a more significant issue.
- Vaginal hysterectomy: Surgeons use scalpels to cut your entire uterus and remove it through your vagina, avoiding the spread of cancerous cells. If your uterus is too big to remove through your vagina, the surgeon will cut it into smaller pieces using a scalpel and remove it.
Since there is no single reliable way to detect uterine sarcoma before surgery, you should talk with your doctor about the safest surgical approach based on your age, health history and cancer risk. In some cases, laparoscopic procedures may still be an option, but only with added precautions like containment systems.
Patient Stories and Advocacy Efforts
Patients and their families have spoken out about their experiences with morcellators and the devastating health impacts of the devices.
Anita Austin underwent laparoscopic power morcellation to remove a fibroid. She was told the approach would be less invasive and offer a quicker recovery. Doctors didn’t mention the risk of spreading cancer even though she had a history of thyroid cancer.
After the procedure, she learned that cancerous cells had been spread in her abdominal cavity, significantly worsening her prognosis.
“The big issue for me is that no one mentioned cancer,” Anita Austin shared. “Not at all. And I question things. I’d had thyroid cancer [in the early ‘80s], and I would have probably done something different. Given my history, honestly, I should have been advised not to do it.”
Austin died of metastatic leiomyosarcoma in October 2016.
Dr. Amy Reed’s story helped bring national attention to the risks of morcellators. After undergoing fibroid surgery in 2013 with a power morcellator, she was diagnosed with LMS. The cancer spread during the procedure, and her prognosis declined sharply.
Her case was the first reported to the FDA as an adverse event despite hundreds of other women experiencing the same issue.
In response, Reed and her husband, Dr. Hooman Noorchashm, began speaking out publicly, urging the FDA and lawmakers to take action. Their efforts played a key role in pushing for stronger safety guidance, manufacturer accountability and greater awareness.
Reed died in 2017 in Pennsylvania. Her legacy continues through the nonprofit she founded, Slay Sarcoma, which focuses on advocacy and funding for LMS research.
In 2014, Viviana Ruscitto underwent morcellator surgery to remove uterine fibroids. She was diagnosed with metastatic leiomyosarcoma days after her procedure and died less than a year later.
Commenting about Viviana’s death, Dr. Noorchashm stated, “Viviana’s death was unnecessary and completely avoidable and the result of the FDA not taking action in the face of clear public health hazards.”
Patient experiences like these have been instrumental in changing how morcellators are viewed and in helping others make more informed decisions about their care.
The Legal and Safety Legacy of Morcellators
Power morcellators were once seen as a breakthrough in minimally invasive surgery. But for many women, these devices caused devastating harm by spreading undetected cancer during routine procedures.
The turning point came in 2014 when the FDA issued its first safety warning. That guidance, driven by patient reports, expert reviews and lawsuits, acknowledged that power morcellators could spread cancer and that the risk was far higher than previously believed.
The morcellator story serves as a cautionary tale in system failure and defective device litigation. Devices entered the market without full clinical testing, early warning signs were overlooked and patients felt the consequences.
If you or a loved one developed cancer after fibroid surgery or a hysterectomy involving a power morcellator, you may still have legal options. Visit our power morcellator lawsuits page to learn more.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.