FDA News & Recalls
-
-
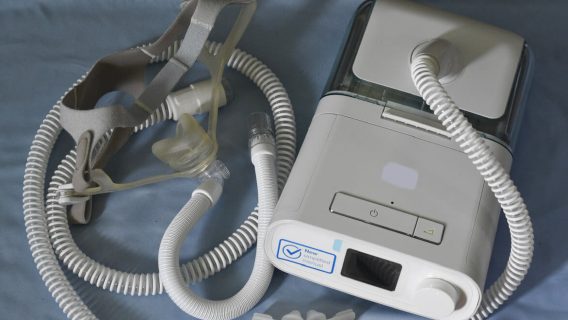 Deaths Linked to Philips CPAP, BiPAP Machines RisingJune 23, 2023
Deaths Linked to Philips CPAP, BiPAP Machines RisingJune 23, 2023 -
 FDA: ‘Triple Shock’ Caused 2022 Baby Formula ShortageJune 22, 2023
FDA: ‘Triple Shock’ Caused 2022 Baby Formula ShortageJune 22, 2023 -
-
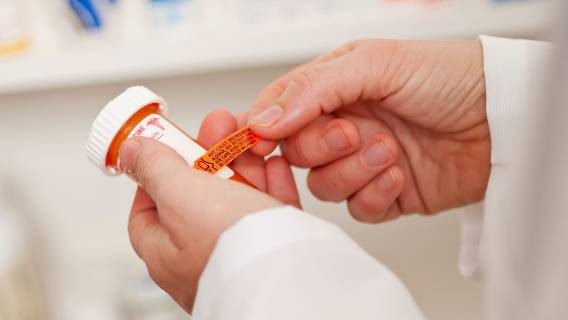 ADHD Medication Will Soon Include Updated Warning LabelsJune 19, 2023
ADHD Medication Will Soon Include Updated Warning LabelsJune 19, 2023 -
Who Am I Calling?
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
(888) 645-1617
