Duodenoscope: Infections, Recalls & Lawsuit Information
Duodenoscopes are a crucial part of ERCP procedures. However, these devices are difficult to sanitize, leading to potential risks like infections and patient deaths. Due to these possible dangers, duodenoscopes have been the subject of litigation and multiple FDA safety warnings.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by Wilson & Peterson LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Kenneth S. Fill, Pharm.D., MBA
- Last update: November 25, 2025
- Est. Read Time: 7 min read
Endoscopic retrograde cholangiopancreatography (ERCP) procedures combine endoscopy and x-ray features to evaluate problems in your bile ducts, gallbladder, liver and pancreas. A duodenoscope is crucial for these procedures because it helps doctors look inside your body.
Duodenoscopes have undergone redesigns over the years to enhance overall safety. However, many patients have experienced serious side effects, including infections and death, due to challenges associated with sanitizing the devices.
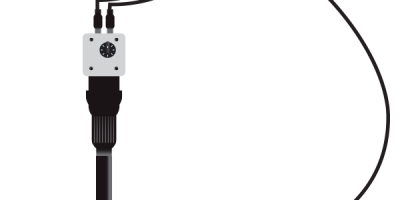
What Is a Duodenoscope and How Is It Used?
A duodenoscope is a lighted, flexible tube that is threaded down your throat, through your stomach and into the top of your small intestine (duodenum). It is used during endoscopic retrograde cholangiopancreatography (ERCP) procedures.
ERCP procedures help diagnose and treat issues with your bile ducts, gallbladder, liver and pancreas. These procedures can be high risk, with complications occurring in up to 10% of patients.
The main danger lies in the design of the duodenoscope. These reusable devices can be difficult to sanitize. Harmful bacteria left behind during the cleaning process can lead to life-threatening infections.
Duodenoscope Infections and Patient Risks
Proper cleaning and disinfecting of duodenoscopes has been a challenge for medical facilities. When not adequately cleaned, duodenoscopes can allow the transfer of tissue or fluid from one patient to another, leading to infection.
However, experts warn that patient-to-patient transmission is rare. Of the estimated one million ERCPs performed between 2013 and 2015, 150 patients developed infections.
The American Gastroenterological Association states, “The therapeutic benefits of [ERCP] far outweigh the potential low risk of infection.”
| Timeframe | Outbreaks | Estimated Patients Affected |
|---|---|---|
| 2014 | 12 | 166 |
| Spring 2015 | 25 minimum | 250 American and European patients |
| January 2000 - December 2017 | 32 | 400 patients across 45 sites (likely underreported) |
Common Infections Reported
Some of the most common infections reported from procedures with duodenoscopes include carbapenem-resistant Enterobacteriaceae (CRE), E. coli and Pseudomonas.
- Carbapenem-resistant Enterobacteriaceae (CRE): These are bacteria that regularly used antibiotics aren’t effective in treating. CRE infections can be deadly.
- E. coli: While E. coli lives in your gut and is usually harmless, infections can occur due to contamination. Mild cases don’t require antibiotics, but certain strains can lead to serious complications like dehydration or organ damage.
- Pseudomonas: These bacteria can impact your blood, lungs, skin and more. Treatment usually consists of antibiotics.
In 2014, roughly 281 patients at Hartford Hospital in Connecticut were exposed to a drug-resistant strain of E. coli after procedures using duodenoscopes. Ronald Reagan UCLA Medical Center alerted 179 patients that they may have been exposed to CRE bacteria after ERCP procedures in 2015
Other medical centers that have reported infections after ERCP procedures using duodenoscopes include the University of Pittsburgh Medical Center and Advocate Lutheran General Hospital in Illinois.
Serious Complications
Severe complications can occur due to the infections caused by duodenoscopes, including sepsis, hospitalization and death.
Researchers have identified cases of sepsis caused by CRE infections after duodenoscope procedures. A May 2024 study in the Journal of Hospital Infection found that sepsis rates after an ERCP procedure using a duodenoscope range from 0.3% to 5.4%.
Studies have also found that some patients needed hospitalization to administer intravenous antibiotics to treat infections after ERCP procedures. Research published in eClinicalMedicine found that 1% of duodenoscope complications may involve hospitalization of 10 days or more.
Alarmingly, the U.S. Food & Drug Administration (FDA) reported 79 deaths between January 2015 and mid-2019 potentially linked to duodenoscopes. Some of these cases involved patients at Seattle’s Virginia Mason Medical Center. The center reported the deaths of 11 out of 32 CRE-infected patients after procedures involving duodenoscopes.
FDA Warnings, Communications and Safety Alerts
The FDA has issued multiple communications about duodenoscopes, their risks and suggestions for minimizing the dangers.
-
Fall 2013:
The Centers for Disease Prevention and Control (CDC) alerted the FDA to a possible link between duodenoscopes and drug-resistant bacteria.
-
February 2015:
The FDA sent a safety communication to health care professionals, alerting them that the complex design of duodenoscopes may impair proper cleaning of the devices.
-
May 2015:
The FDA organized the Gastroenterology-Urology Devices Panel of the Medical Devices Advisory Committee to discuss minimizing the risks of duodenoscopes.
-
August 2015:
A safety communication was sent by the FDA with additional protocols to improve the cleaning of duodenoscopes.
-
October 2015:
The FDA required duodenoscope manufacturers (Fujifilm Medical Systems USA, Inc., Olympus Medical Systems Corporation and Pentax of America) to conduct postmarket surveillance about how the devices are prepared for reuse.
-
January 2016:
The TJF-Q180V duodenoscope from Olympus received approval from the FDA with changes to its design that were intended to reduce the risk of infection.
-
January 2017:
A safety communication was issued by the FDA to enhance awareness that Fujifilm removed certain older duodenoscope models from the market.
-
September 2017:
The FDA approved the Pentax ED34-i10T duodenoscope with a disposable cap to improve cleaning and reuse.
-
February 2018:
The FDA, CDC and American Society for Microbiology established voluntary, standardized protocols for duodenoscope surveillance.
-
March 2018:
Warning letters were sent to the three duodenoscope manufacturers in the U.S. for not providing adequate data to fulfill the postmarket surveillance studies requirements. The manufacturers responded by submitting plans of how study milestones will be met.
-
December 2018:
The FDA released interim results from the ongoing postmarket duodenoscope surveillance studies. Results showed higher-than-anticipated contamination rates after disinfection.
-
April 2019:
The FDA issued a safety communication reminding medical facilities to follow duodenoscope sanitization and maintenance instructions, adhere to best practices and report adverse events to the FDA.
-
August 2019:
A safety communication was provided by the FDA encouraging medical facilities to transition to duodenoscopes with innovative designs that minimize infection risks. A recommendation was also provided for Fujifilm, Pentax and Olympus to remove fixed endcap duodenoscopes from circulation.
-
November 2019:
The Medical Devices Advisory Committee met to seek expert opinions about duodenoscope reprocessing based on current scientific data.
-
December 2019:
The first fully disposable duodenoscope was approved by the FDA. It was the first duodenoscope created by manufacturer Boston Scientific.
-
April 2022:
The FDA sent a safety communication in support of transitioning to fully disposable duodenoscopes.
-
June 2022:
The April safety communication from the FDA was updated, reinforcing that using newer models of duodenoscopes can reduce the risk of patient infections compared to older models.
The FDA’s most recent stance is that health care providers and facilities should switch to disposable duodenoscopes to reduce the risk of patient infection.
Recalls and Manufacturer Actions
Duodenoscope manufacturers have been actively involved in device research, recalling faulty models and replacing them with improved products.
Fujifilm, Olympus and Pentax have all issued class two recalls for issues pertaining to defective designs of their duodenoscopes. Issues cited in the various recalls include:
- Instruction updates
- Mechanism replacements
- Potential injuries associated with parts of the duodenoscope detaching
- Risk of contamination or infection
- Suction button becoming stuck
In 2017, the FDA approved the Pentax ED34-i10T duodenoscope. This device had a disposable cap, allowing for improved sanitization. The FDA later cleared the first fully disposable duodenoscope, the EXALT Model D Single-Use Duodenoscope from Boston Scientific, in 2019.
Postmarket surveillance found that using partially or fully disposable duodenoscopes can reduce contamination risk by 50% or more. While this is an improvement, it indicates that some risks remain despite design updates to duodenoscopes.
- Collaborate with medical and federal partners to evaluate new strategies for lowering duodenoscope infection rates.
- Continue evaluating and improving reprocessing methods.
- Encourage design innovation beyond fixed endcap duodenoscopes to a more modern design that facilitates or eliminates reprocessing.
- Evaluate available data about duodenoscope complications from various sources.
- Look at current duodenoscope reprocessing instructions to determine their effectiveness.
- Share suggestions with health care providers to lower the risk of infection transmission.
- Work with industry professionals to modify and validate reprocessing instructions to enhance the duodenoscope sanitization and safety.
Olympus and Other Manufacturers Under Scrutiny
Fujifilm, Olympus and Pentax have all received warning letters from the FDA. Issues addressed in the letters included failing to meet good manufacturing practice requirements and not completing required reporting related to their duodenoscopes
However, as the largest duodenoscope manufacturer, Olympus has gotten the most attention.
Olympus has received multiple warning letters from the FDA, along with a letter from the U.S. Senate on Health, Labor, Education & Pensions in 2015. The letter, written by Ranking Member Patty Murray, questioned Olympus’ failure to issue “important safety advice” in the U.S. despite providing warnings in Europe.
In 2023, Olympus and one of its subsidiaries received additional warning letters after FDA facility inspections in Japan. The letters cited the manufacturer’s failure to complete reporting and violations of quality regulations.
Most recently, the FDA issued a June 2025 alert about Olympus products manufactured in Japan. Due to compliance issues, the FDA was working to prevent certain devices from being shipped to the U.S. This alert indicates a pattern of regulatory violations from the manufacturer.
Legal Basis and Outlook for Duodenoscope Lawsuits
Patients and families adversely impacted by duodenoscopes have filed duodenoscope lawsuits.
Allegations against manufacturers include:
- Failure to warn hospitals and patients about infection risks
- Ignoring FDA orders for safety testing
- Selling defective scopes with a flawed cleaning design
The first duodenoscope lawsuits were filed in 2015 after the infections at Ronald Reagan UCLA Medical Center came to light. These cases likely contributed to FDA warnings and the eventual push for safer disposable duodenoscopes.
Today, reusable duodenoscopes remain on the market, so the risk to patients is still present.
Many lawyers are still accepting duodenoscope cases and offer a free consultation so you can discuss your options. If you were harmed by a duodenoscope, talk to an experienced medical attorney to find out if you are eligible to file a lawsuit.
Transition to Safer Alternatives
In 2019, the FDA made an official recommendation for medical facilities to upgrade their duodenoscopes. The FDA asserted the importance of utilizing “innovative device designs that make reprocessing easier, more effective or unnecessary.”
By 2021, the FDA had cleared six disposable duodenoscopes, including models from Boston Scientific Corporation, Fujifilm, Olympus and Pentax.
Despite the decreased risk of infection, adoption of disposable duodenoscopes has been slow. Issues like cost have proven to be a challenge in terms of getting medical facilities to use safer versions of these devices.
One study found that it could cost hospitals an extra $367,200 over a three-year period to switch to disposable duodenoscopes. This number may increase for lower-volume health care facilities.
Studies will likely continue as duodenoscopes evolve to meet patient needs. Still, manufacturers have made significant improvements in the safety of their devices, which has helped lower infection rates.
Patient Safety and What To Do if Harmed
ERCP procedures carry several risks that can be further complicated by the use of duodenoscopes. If you experience any side effects, such as fever, chills or abdominal pain, the first thing you should do is talk to your doctor.
- Abdominal pain
- Bleeding, drainage, redness or swelling from your IV site
- Bloody, black or tarry stools
- Difficult swallowing
- Fever or chills
- Increasing pain
- Nausea or vomiting
These symptoms can become life-threatening without medical attention, so do not wait to alert your doctor if you experience any of these complications.
Be sure to keep records of your procedures, doctor’s visits, complications, diagnoses and other relevant information if you are harmed during an ERCP procedure. Having this information readily available can be helpful if you choose to file a lawsuit.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.