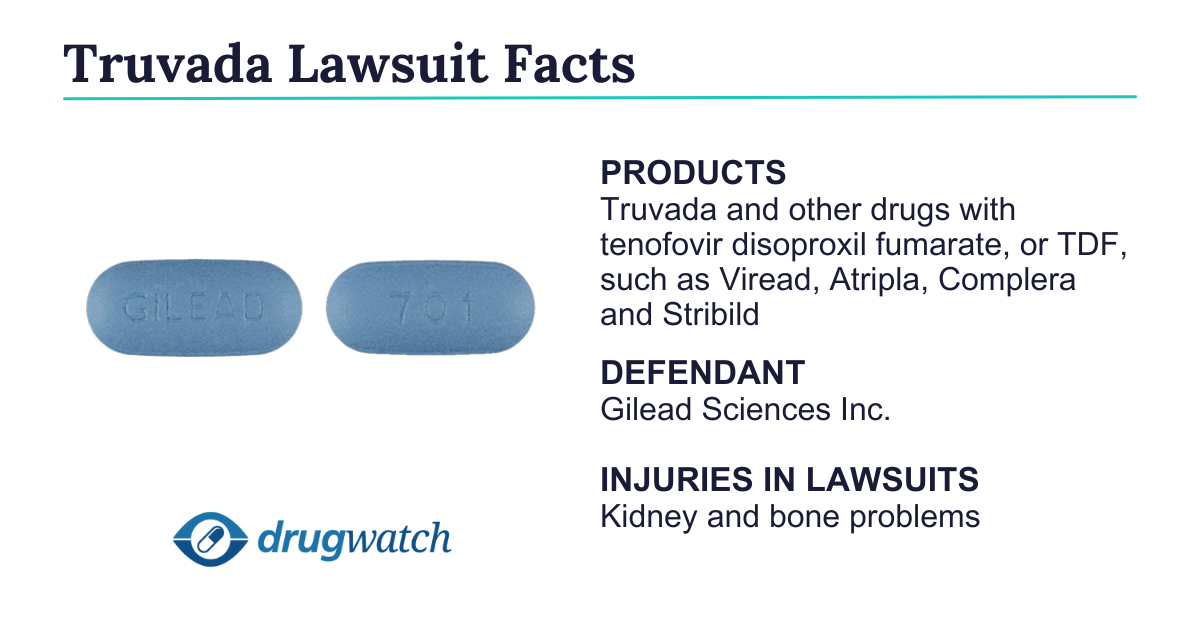
Truvada Lawsuits
Truvada (emtricitabine and tenofovir disoproxil fumarate) lawsuits claim that the drug causes kidney problems and bone loss. Truvada lawsuits also claim that Gilead withheld a safer alternative to maximize profits from Truvada. Gilead offered a $40 million settlement in June 2024.
Article Continues Below
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- MDL
- No MDL, but a group of cases are consolidated in the Northern District of California under JCCP No. 5043
- Settlements
- $40 million in June 2024
Latest Truvada Lawsuit Updates
As of October 2024, lawyers continue to accept potential Truvada lawsuits. This litigation also includes Viread, Atripla, Complera and Stribild lawsuits because all these drugs contain tenofovir disoproxil fumarate (TDF), an ingredient that plaintiffs argue is toxic to the bones and kidneys.
We’ve spoken to lawyers familiar with the litigation and analyzed court documents and news publications to bring you the following updates.
-
October 2024:
There have not been any major updates over the last few months as we continue to await word on the completion of Gilead's $40 million settlement offer. Lawyers are still accepting new Truvada cases.
-
July 2024:
While Gilead offered $40 million last month to settle more than 2,600 cases in California federal court, the drugmaker faced about 25,000 active cases in state or federal courts in California and Missouri, according to our review of Gilead’s last quarterly report. There has been no word on how close these cases are to resolution. The first bellwether trial in California federal court was rescheduled for November 2024, but with the settlement offer, trial proceedings are likely stayed until further notice.
-
June 2024:
Gilead offered $40 million to settle approximately 2,625 TDF cases in California federal court. These plaintiffs claimed that TDF is dangerous and caused unnecessary bone and kidney problems because Gilead withheld a safer drug and failed to warn about the risks. The drugmaker said it “entered into this agreement to avoid the cost and distraction of litigating these cases, and in no way is this settlement an admission of liability or wrongdoing.” As part of the terms of the settlement agreement, at least 98% of claimants must opt-in.
-
January 2024:
Federal consolidated Truvada HIV lawsuits in California were still pending. The federal judge overseeing consolidated federal cases in California dismissed some claims but preserved others in the Gilead litigation. The first federal trial was scheduled for April 2024.
-
November 2023:
In a price-fixing class action, Gilead reached a $246.75 million preliminary settlement with plaintiffs claiming that the company conspired with a drug manufacturer to delay the release of generic versions of their HIV drugs. The settlement addresses claims related to price-fixing, and a final approval hearing was expected in January 2024.
-
September 2023:
A California appeals court heard arguments in state-consolidated lawsuits on Gilead’s appeal of the denial of a motion for summary judgment. The motion claimed the company fraudulently or negligently withheld a safer HIV drug for almost a decade to maximize its profits.
-
May 2023:
The first of two summary jury trials began. These summary trials are nonbinding and both sides present their cases in a private, unrecorded setting.
-
May 2023:
A group of 19 plaintiffs filed a lawsuit against Gilead in the Northern District of California alleging Gilead failed to warn about tenofovir disoproxil fumarate’s toxicity to the bones and kidneys.
-
April 2023:
Plaintiffs urged U.S. District Judge Jon S. Tigar of the Northern District of California to reject Gilead’s request to dismiss approximately 650 plaintiffs who supposedly had not provided information to back their claims.
-
March 2023:
Judge Tigar tossed out eight of Gilead’s experts after he concluded their opinions were the same and probably ghostwritten by Gilead’s lawyers. Both sides continued to file motions to exclude expert witnesses.
-
December 2022:
Gilead reported the company faced lawsuits involving more than 26,000 plaintiffs in California, Delaware, Florida, Missouri and New York.
According to Gilead’s March 2024 quarterly report, the first bellwether test trials were scheduled in California state and federal courts for October 2022 but have since been stayed. Many of the cases are pending in California, but the company faces lawsuits that involve thousands of plaintiffs in multiple states.

Do I Qualify to File a Truvada Lawsuit?
You may qualify to file a Truvada or TDF lawsuit if you took Truvada or another TDF medication manufactured by Gilead and suffered bone loss or kidney damage.
Truvada lawsuits claim that Gilead knew or should have known that Truvada and its active ingredient TDF were “highly toxic in the doses prescribed and risked permanent and possibly fatal damage to the kidneys and bones.”
“Gilead has shown a disregard for its patients’ health in order to reap outsized profits from its TDF medications.”
However, instead of conducting further studies and informing the public, the drugmaker ignored and misrepresented the risks, according to the combined lawsuit of Michael Lujano and Jonathan C. Gary. Gilead hid the risks from doctors and patients so it could “continue to raise its market share with TDF.”
“Gilead has shown a disregard for its patients’ health in order to reap outsized profits from its TDF medications,” Lujano told the Associated Press.
Over the years, several studies have linked TDF to side effects that include kidney and bone problems.
Kidney Problems
The first documented instance of kidney toxicity in relation to TDF occurred in the United States the same year the drug hit the market in 2001, according to Willem D.F. Venter and the coauthors of a 2018 study in Southern African Journal of HIV Medicine.
The patient suffered acute kidney injury, nephrogenic diabetes insipidus and Fanconi syndrome, a disorder where certain substances normally absorbed into the blood are excreted in urine instead.
“Since then, many studies have described the nephrotoxic potential of the drug,” the authors wrote.
- Acute kidney injury or acute renal failure
- Chronic kidney disease or declining kidney function
- Fanconi’s syndrome
- Kidney tubular dysfunction
Bone Density Loss
A 2018 study by Grace A. McComsey and colleagues published in AIDS found loss of bone mineral density “in patients with HIV is associated with many contributing factors including antiretroviral therapy (ART), particularly regimens including tenofovir disoproxil fumarate.”
Researchers found switching to a combination of two other antiretroviral drugs, dolutegravir and rilpivirine, continued to suppress the virus while improving bone mineral density.
- Osteopenia
- Osteoporosis
- Bone fractures
People Who Filed Truvada Lawsuits
People filed the first individual Truvada injury lawsuits in California in May 2018, and others followed. The common assertion in these claims is that Gilead failed to warn about the drug’s side effects and purposely withheld the safer drug tenofovir alafenamide (TAF), now marketed as Descovy, from the market to make more money.
Gilead’s sales reps and CEO claimed TDF was “a ‘miracle drug,’ had ‘no toxicities,’ was ‘benign,’ and ‘extremely safe’,” according to lawsuits. However, studies as far back as 1997 showed it had significant bone and kidney toxicity.
Michael Lujano and Jonathan C. Gary v. Gilead
Michael Lujano and Jonathan C. Gary took Truvada and Atripla for several years. In May 2018, they filed their lawsuit in California state court.
TDF Usage
Lujano took Truvada from 2004 to 2009. Then, he took Atripla from 2009 to 2015. Gary took Truvada from 2001 until 2011.
Injuries Claimed
Doctors diagnosed Lujano with osteopenia and osteoporosis of the spine, neck and hip. Doctors diagnosed Gary with Fanconi syndrome, osteopenia and osteoporosis.
Relief Sought
The lawsuit sought general damages, past and future medical expenses and punitive damages.
Gilead tried to have the case dismissed, but in February 2019, Judge Carolyn B. Kuhl allowed the claims to continue on all actions except strict liability. The case was allowed to proceed on negligence and breach of warranty claims.
Christopher Pierot v. Gilead
Christopher Pierot filed his case in July 2018 in Louisiana federal court.
TDF Usage
Pierot took Truvada to manage his HIV from 2008 to 2009.
Injuries Claimed
Pierot was 26 when he started taking Truvada. By 30, he suffered bone necrosis and bone loss so severe that he needed to have both hips replaced. He suffered a great deal of pain after each surgery.
Relief Sought
The lawsuit sought general damages, past and future medical expenses and punitive damages.
Pierot’s lawsuit claimed Gilead’s studies showed levels of TAF in target cells were about 10-fold and 30-fold greater than with TDF. TAF was also more efficient and barely detectable after the body used it. But TDF remained in blood plasma, a marker that it could be toxic to non-target cells. Despite this knowledge, Gilead continued to sell the less effective, more toxic TDF and withhold TAF from patients.
Vanessa L. Naisha v. Gilead
Vanessa L. Naisha filed a Truvada lawsuit against Gilead in Delaware on July 11, 2019.
TDF Usage
Doctors diagnosed Naisha with HIV in 1986, and she began taking Truvada in 2004.
Injuries Claimed
By 2009, Naisha experienced severe pain in her hips and loss of balance. In 2010, she had to use a wheelchair after her hips failed. She had two hip replacement surgeries in 2011 and suffered complications during the second surgery. She had to go to the intensive care unit for a blood transfusion and did not regain consciousness until hospital staff ran electricity through her body seven times.
Doctors said her bone mineral density was osteopenic. It wasn’t until 2018 that she discovered Truvada might be to blame.
Relief Sought
Naisha sought relief in the amount of $10 million for pain and suffering.
Naisha “put her trust in [the] Defendant with hopes of getting treatment for HIV. She was oblivious to the fact that all these years she was ingesting poison,” the complaint said.
AIDS Health Foundation Files Truvada Class Action Lawsuit
AIDS Health Foundation (AHF), the largest global AIDS organization, helped file a class action lawsuit against Gilead on behalf of Devin Martinez, Ricardo Wohler and others who purchased Truvada and other TDF drugs in May 2018.
This California action is separate from the individual injury lawsuits filed by people who claim kidney or bone injuries from TDF, but it deals with similar issues.
- Failure to disclose TDF carried a significant risk for toxicity and bone and kidney damage
- Failure to warn of kidney and bone risks in all patients, not just those with preexisting kidney and bone problems
- Misrepresentation of the risks and benefits of TDF drugs
According to the complaint, Gilead misrepresented TDF’s safety profile as early as 2001. The FDA reprimanded Gilead in 2002 and 2003 for claiming the drug had no toxicities and would not cause bone or kidney damage, but the company continued to downplay the risks.
Gilead’s ‘Bald-Faced Greed and Disregard for Patient Safety’
Before the class action, AHF had sued Gilead in April 2016. The foundation accused the company of manipulating the patent system by withholding TAF from patients.
AHF said in a press release that media coverage on the topic had “exposed Gilead’s bald-faced greed and disregard for patient safety.”
The lawsuit alleged that as early as 2002, Gilead was paying doctors to research TAF in patients around the country while it was making profits on TDF. The company quietly applied for patents on TAF but kept its research secret.
In response to the allegations, Gilead’s lawyers denied lawsuit claims and said the company “had no duty to develop, test, seek approval of, or launch its new product on any particular timetable,” the Los Angeles Times reported.
“The delay in conducting clinical trials deprived those suffering from HIV of TAF for more than a decade,” the class action lawsuit said. “It is possible that HIV patients suffered from 10 years of additional accumulated kidney and bone toxicity using TDF while TAF stayed on the shelf.”
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.





