Elmiron Lawsuits
Elmiron lawsuits claim people suffered serious vision damage after taking the drug. As of July 2025, there were 902 lawsuits pending in a federal MDL. Some Elmiron lawsuits have confidentially settled in 2023. Drugwatch's legal partners are currently not accepting new Elmiron lawsuits.
- Legally reviewed by Trent B. Miracle, Esquire
- Last update: July 1, 2025
- Defendant
- Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson
- Injuries in Lawsuits
- Maculopathy, retinopathy and other vision problems
- MDL
- U.S. District Court of New Jersey before Judge Brian R. Martinotti MDL 2973, 2:20-md-2973
- Settlements
- Some cases have confidentially settled
Latest Elmiron Lawsuit Updates
As of July 2025, there are 902 Elmiron cases pending in multidistrict litigation in the U.S. District Court of New Jersey before Judge Brian R. Martinotti. A total of 1,986 lawsuits have been filed in the MDL. Settlements are expected in MDL 2973.
The litigation is ending and could settle soon. Our legal partners are no longer accepting these cases. We’ve spoken to lawyers familiar with this litigation and reviewed court documents to bring you these updates.
-
July 2024:
While Janssen has been quietly settling some cases, more than 1,500 are still pending in the MDL in New Jersey. Last month, lawyers for both sides met with Francis Yook on behalf of Special Master Mark Falk to discuss further settlements. A status report is due 60 days from June 20, 2024.
-
May 2024:
Cases continued to settle confidentially, and MDL numbers slowly decrease as the litigation winds down. Judge Martinotti cancelled a settlement conference that was supposed to be held May 13 and hasn't rescheduled it yet.
-
November 2023:
Lawyers were still accepting new cases. Some cases have been settled confidentially.
-
March 2023:
The first bellwether trial had been scheduled to start at the end of the month. Some lawyers thought that trials were paused as Elmiron manufacturer Janssen Pharmaceuticals negotiates settlements.
-
October 2022:
Judge Martinotti rescheduled the first bellwether trial, Maria Windham v. Janssen Pharmaceuticals, Inc., et al., 20-cv-14670, to March 27, 2023. There hadn't been a ruling on the other bellwethers, but these dates were likely moved down to accommodate the rescheduling of the first trial.
-
September 2022:
The judge scheduled the first bellwether trial in the Elmiron MDL for January 2023. A second trial was scheduled to begin in March 2023 and a third was to follow in May 2023.
-
July 2022:
Judge Brian Martinotti approved 14 plaintiffs' attorneys for the Plaintiffs' Steering Committee, a group of attorneys who oversee and coordinate the litigation for the plaintiffs in the MDL.
Researchers published the first studies linking Elmiron to pigmentary maculopathy in 2018. In 2020, Janssen updated the drug label to include a warning for “changes in the retina of the eye (pigmentary maculopathy).” In 2020, shortly after adding the warning to the label, plaintiffs filed the first Elmiron lawsuits.
The first bellwether trial in the Elmiron multidistrict litigation in New Jersey, Windham v. Janssen Pharmaceuticals, Inc., et al. 2:20-cv-14670, was scheduled for January 2023 and was later moved to March 27, 2023. In April 2023, the parties jointly agreed to withdraw all motions, presumably for settlement talks.

Award-winning mass torts and product liability lawyer Trent B. Miracle answers three important questions about Elmiron lawsuits.
- Elmiron litigation seems close to ending. What made Elmiron litigation unique from other mass torts?
Elmiron litigation is a few years old at this point. This is a unique case because typically these MDLs move extremely slowly. But we had gotten a lot of the legwork done before the MDL was assigned. The first trial in that case was supposed to happen back in March 2023, and it got postponed.
- What were some of the injuries claimed in lawsuits?
Specifically, some of the injuries [that were] named in these suits include retinal pigmentary maculopathy and macular degeneration.
- What were some of the symptoms lawyers screened for in these lawsuits?
Generally, we screened potential cases for the following symptoms: Blurred or distorted vision, difficulty reading, loss of vision, eye pain, pigmentary maculopathy, trouble adjusting to darkness, vision disturbances and macular degeneration.
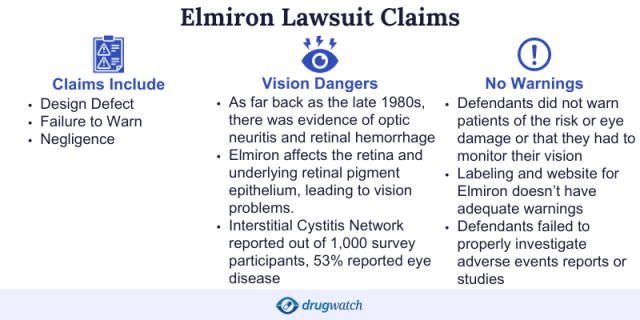
What Did Elmiron Lawsuits Claim?
Elmiron lawsuits were filed against Janssen Pharmaceuticals by plaintiffs who suffered vision problems that could lead to blindness, including maculopathy.
“[Patient’s] lives have been profoundly changed by this. They were taking something they thought was just going to help their day-to-day quality of life because they were in pain. And now, they can’t do things that they took for granted — like just sitting down and reading a book — because they've got maculopathy. The middle of their field of vision could be gone.”
Plaintiffs claimed Elmiron, a medication used to treat interstitial cystitis, caused their vision damage and that it is a dangerously defective drug. According to lawsuits, Janssen withheld adverse event reports from the public, medical community and the U.S. Food and Drug Administration.
The lawsuits called for Janssen to pay compensation for permanent vision injuries.
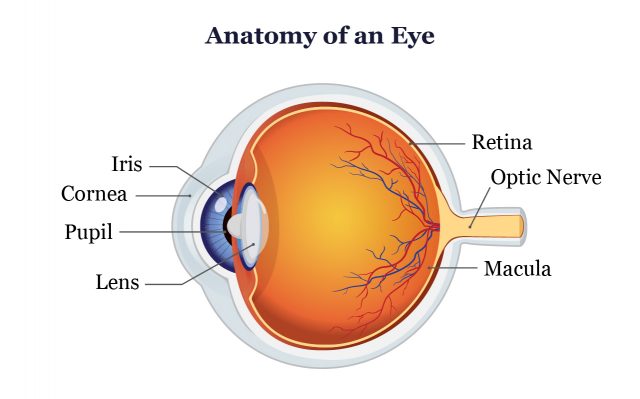
Elmiron Linked to Maculopathy
Several studies have linked Elmiron, the brand name of pentosan polysulfate sodium (PPS), to a unique type of maculopathy that only occurs in Elmiron users called pigmentary maculopathy. This type of maculopathy could lead to blindness and there is no cure.
“In 2018, eye physicians published the first report of retinal damage associated with Elmiron use,” Miracle told Drugwatch. “Since then, the medical literature regarding eye damage and vision loss associated with Elmiron continues to grow.”
“[Patient’s] lives have been profoundly changed by this. They were taking something they thought was just going to help their day-to-day quality of life because they were in pain. And now, they can’t do things that they took for granted — like just sitting down and reading a book — because they’ve got maculopathy. The middle of their field of vision could be gone,” Miracle said.
People Who Filed Elmiron Lawsuits
People began filing lawsuits against Janssen in 2020. Three of the earlier lawsuits belonged to Jeanette Milburn, Valarie Hull and Kimberly Pelczar. The three women claimed long-term Elmiron use caused them to develop loss of night vision, pigmentary maculopathy, vision degradation, retinal injury and retinal hemorrhage.
They claimed Elmiron was defective and that Janssen failed to warn them and their doctors of the risk of permanent vision damage.
Elmiron Case Study
Samantha Padelford v. Jannsen Pharmaceuticals et al.
Samantha Padelford filed one of the more recent Elmiron lawsuits in 2023.
Elmiron Usage:
Padelford took Elmiron from 2001 to 2008 for interstitial cyctitis, and she claimed she nor her physician were warned about the risks of vision damage.
Injuries Claimed:
She suffered from “substantially impaired vision, among other symptoms.” Padelford claimed she suffred bodily injury, pain and suffering, disability, mental anguish, loss of capacity for enjoyment of life lost earnings and aggravation of previously existing conditions.
Relief Sought:
Compensatory damages, statutory damages, punitive damages, attorneys’ fees and costs.
Elmiron Lawsuit Settlement Amounts
In 2023, Janssen and Johnson & Johnson started quietly settling some Elmiron lawsuits, although they have not publicly disclosed any official settlement amounts. Some lawyers estimate that Elmiron lawsuit settlement amounts could reach hundreds of thousands of dollars.
Because there have been no trials, jury verdicts or global Elmiron settlement offers, determining the potential Elmiron lawsuit settlement amounts is challenging. Bellwether trial verdicts usually serve as a benchmark for the value of these cases.
Courts scheduled the first Elmiron bellwether trial for March 2023 but later canceled and never rescheduled it as attorneys continue to work on a potential global settlement.
Will There Be an Elmiron Recall?
There has not been an Elmiron recall, despite studies linking the long-term use of the interstitial cystitis drug to potentially permanent vision damage.
The drug has been on the market since 1996 and is still the only oral treatment available for the condition. Janssen didn’t warn about the risk of retinal pigmentary maculopathy until June 2020.
The FDA published the new label with the maculopathy warning but has not issued a statement or safety communication about it. So far, the Agency hasn’t asked Janssen to recall the drug and the drugmaker hasn’t said it plans to issue a recall.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.
