Elmiron
Elmiron (pentosan polysulfate sodium), is a weak blood thinner used to treat discomfort or bladder pain associated with interstitial cystitis, a chronic condition. The most common side effects of Elmiron are hair loss, diarrhea and nausea. Headache, abdominal pain and upset stomach may also occur.
What Is Elmiron?
Elmiron is a brand-name, prescription-only form of the medication pentosan polysulfate sodium. Medical professionals such as urologists and urogynecologists commonly prescribe it to treat the pain and discomfort of interstitial cystitis when dietary modifications have not provided relief. IC causes a range of symptoms, including bladder pain and pressure that worsen as the bladder fills, frequent urination, pain when urinating and urinary urgency (feeling a constant urge to urinate).
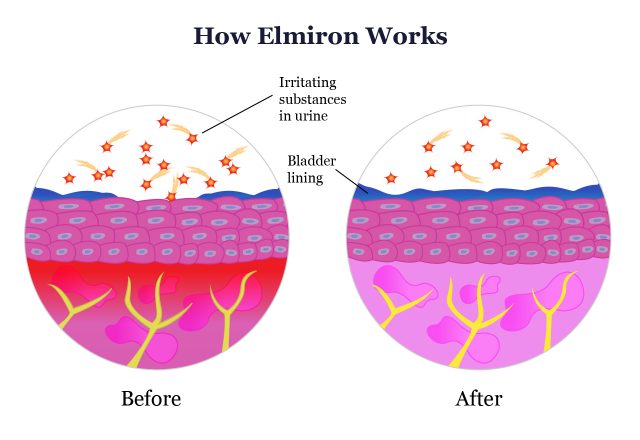
Researchers don’t completely understand the action of pentosan polysulfate in the body or how it protects the bladder from recurring IC symptoms. Elmiron isn’t a pain reliever like acetaminophen or a nonsteroidal anti-inflammatory drug like ibuprofen. Instead, it may work by creating a barrier against acidic urine that can irritate an already damaged bladder lining.
It’s important to note that Elmiron is not a cure for IC, but it does treat many bothersome symptoms of this chronic condition. Over time, you may be able to return to normal activities as your bladder function becomes more manageable.
Elmiron Side Effects
Doctors originally thought that Elmiron side effects only included mild symptoms such as hair loss, diarrhea and nausea. However, researchers are finding that this drug has more significant side effects than previously thought, including vision issues.
Most of the side effects from Elmiron are mild. However, they can be difficult to deal with if they interfere with your daily life. Consider these common side effects of Elmiron if you’ve taken or are planning to take this drug:
- Abnormal liver function tests
- Blood in the stool
- Bruising
- Diarrhea
- Dizziness
- Hair loss
- Headache
- Skin rash
More unusual side effects reported include insomnia and worsening mood. Most patients in relevant studies have experienced hair loss while taking Elmiron. Although this side effect is not medically dangerous, it can be disheartening. This type of hair loss typically ceases when you stop taking the drug.
Some people reported eye issues such as maculopathy after years of taking Elmiron. Recent studies are confirming that years-long use of Elmiron likely contributes to pigmentary maculopathy.
Elmiron Dosages and Precautions
Doctors currently recommend taking Elmiron three times daily with water on an empty stomach. In total, you will typically take three 100-milligram pills per day, with one pill taken roughly an hour before each meal or two hours after each meal.
It takes a while for Elmiron to start working. Your doctor will ask you to schedule an appointment for three months after you begin taking the drug so that you can evaluate how it is working and whether you need to change treatments.
Make sure to tell your doctor if you are taking blood thinners, aspirin or nonsteroidal anti-inflammatory drugs, as these substances can increase your risk of bleeding. Additionally, inform your doctor of any other substances you take, such as herbal medications or homeopathic tablets, before beginning Elmiron.
Precautions
Your doctor will inform you of Elmiron side effects and how the drug may interact with any other medical conditions you have (other than IC). If you have any of the following conditions, you should be especially careful when taking Elmiron:
- Bleeding Disorders: While Elmiron doesn't have any known drug interactions, it is a mild anticoagulant (blood thinner) that could potentially interfere with how well your blood clots.
- Breastfeeding: It's not known whether Elmiron can pass through breast milk to a nursing infant, so be on the safe side and avoid it if you're breastfeeding.
- Elevated Liver Enzymes: Some studies have shown mildly elevated liver enzymes after using Elmiron for a few months, so be careful taking the drug if you have a history of liver problems.
- Pigmentary Maculopathy: This eye condition sometimes occurs in patients who use Elmiron for three years or longer, so it's important to discuss these potential issues with your doctor.
- Pregnancy: There haven't been well-controlled studies of Elmiron with pregnant women, so avoid using it when you are pregnant.
Not every patient will have a severe reaction to Elmiron. Some may not have Elmiron side effects at all. However, it is impossible to predict what will happen to every individual who takes this drug. Discuss your options with your doctor before taking Elmiron if you have one of the conditions listed here.
Elmiron Lawsuits for Eye Damage
When Elmiron was first developed, its manufacturer, Janssen, did not include a warning about the potential for the drug to cause pigmentary maculopathy or any other eye issues. Many lawyers are now filing Elmiron lawsuits on behalf of people who took the drug and later experienced eye problems. Pigmentary maculopathy may be mistaken for macular degeneration, and it’s often missed at first due to it only exhibiting milder symptoms such as blurred vision.
Janssen added a warning about these issues in June 2020. While additional studies are being performed to confirm whether Elmiron is the true cause of these eye issues, it’s important to know this information if you plan to take the drug. Additionally, if you’ve taken Elmiron for years and you’re experiencing eye symptoms, make sure to discuss these issues with your doctor and optometrist.
Lawyers are currently filing Elmiron lawsuits on behalf of people who took the drug and experienced vision problems that include blurred vision, maculopathy, retinopathy and vision impairment.
Elmiron Alternatives
If you struggle with diagnosed interstitial cystitis or symptoms similar to this condition, it’s important to discuss any Elmiron alternative with your doctor. Elmiron is currently the only U.S. Food and Drug Administration-approved medication that is specifically targeted toward IC. Do not take “generic” alternatives to Elmiron that you may find online. These are not legal, and they might not even be the same drug. Consider these alternatives instead:
- Botox Injections: Some patients find local botox injections helpful for alleviating the pain of IC.
- Diet Modifications: If you have IC, you may notice that the same foods bother your bladder every time you ingest them. Bladder-irritating foods might include coffee, tea, tomato products, spicy foods and chocolate. Bladder-soothing foods typically include dairy products, most fruits and vegetables (except for tomatoes and hot peppers) and grains.
- Dimethyl Sulfoxide: This drug often helps reduce symptoms of IC. If your doctor decides that this treatment is right for you, you can elect to have DMSO administered directly into your bladder with a catheter.
- Ulcer Cauterization: Many people with IC develop painful ulcers on their bladder walls. Cauterization can help reduce symptoms for patients with severe symptoms related to ulcers.
Many patients find that they can control their symptoms through medication and specific lifestyle changes. Talk to your doctor about alternatives to Elmiron if you can no longer take it or are uncomfortable with its side effects.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.



