Overview of the Safe Medical Devices Act of 1990
More than 1.7 million injuries and nearly 83,000 deaths can be linked to medical devices over the past 10 years. Fewer than 0.5% of implanted devices have likely received testing through intensive clinical trials. The Safe Medical Devices Act of 1990 helped the FDA improve its oversight of these products.
- Last update: September 5, 2023
What Is the Safe Medical Devices Act?
The Safe Medical Devices Act of 1990 created important reporting requirements for the use of medical devices. It mandated that manufacturers and health care professionals inform the U.S. Food and Drug Administration of any adverse effects, such as death or injury, associated with a medical device.
- Monitor medical device products after they are cleared for market.
- Track medical devices to ensure traceability of certain devices down to the user level.
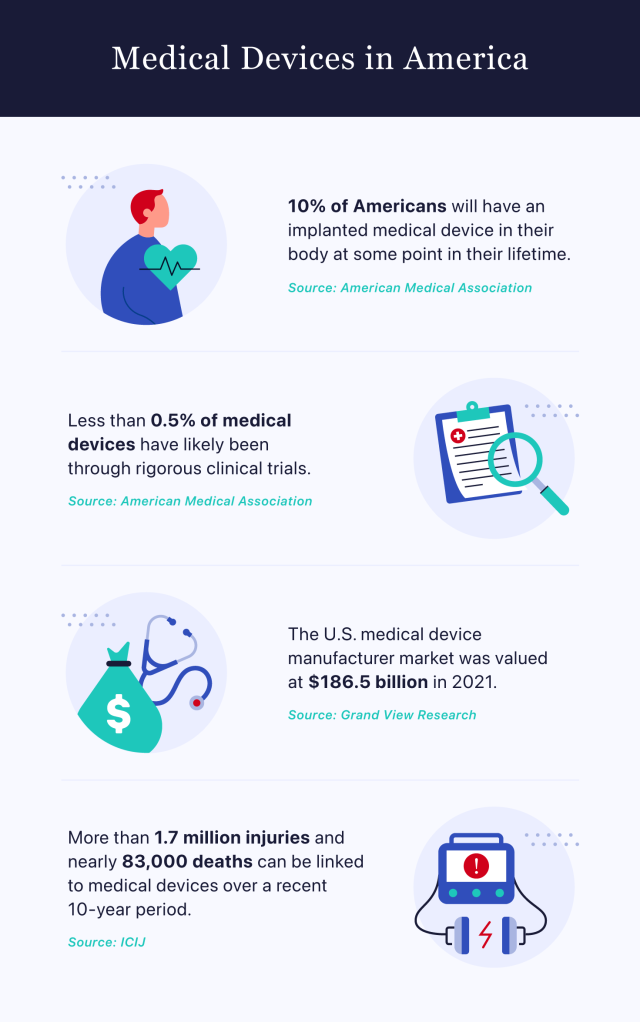
The American Medical Association reports that approximately 10% of Americans will undergo a medical device implantation at some point in their lifetime. It estimates that fewer than 0.5% of these devices will have been tested through intensive clinical trials.
Data gathered by the International Consortium of Investigative Journalists reveals that more than 1.7 million injuries and nearly 83,000 deaths can be linked to medical devices over a recent 10-year period.
Under the SMDA, it became mandatory for health care facilities, device manufacturers and product importers to submit medical device reporting paperwork to the FDA once they became aware of any potential negative incidents associated with a device.
It also explicitly required manufacturers to submit baseline reports and monthly reports to the FDA chronicling any deaths, serious injuries or malfunctions associated with the device.
Why Was the SMDA Created?
Prior to creating the Safe Medical Devices Act, Congress conducted a series of hearings and studies to review existing FDA regulations on medical devices (part of the Federal Food, Drug and Cosmetic Act, or FD&C Act) that had been in place since 1976. They determined that the FDA “received less than adequate information about problems with medical devices.” In an effort to better protect public health from hazardous medical devices, Congress passed the SMDA to guarantee that the FDA would receive better information.
- Ensure medical devices placed on the market are safe and effective.
- Accelerate reporting of adverse events to the FDA.
- Rapidly remove defective products from the marketplace.
All of the SMDA reports submitted to the FDA are added to its medical device reporting database — a searchable resource that allows for quick access to details on all adverse events reported for devices on the market.
How Does the SMDA Define Medical Devices?
Medical devices, as regulated by the FDA, were originally defined in the FD&C Act as:
“Any instrument, machine, contrivance, implant, in vitro reagent that's intended to treat, cure, prevent, mitigate, diagnose disease in man.”
The definition is quite broad, meaning a wide variety of products can be classified as a medical device. Under the auspices of the FD&C Act, medical devices are categorized into three classes based on the device’s inherent risks.
Class I Devices
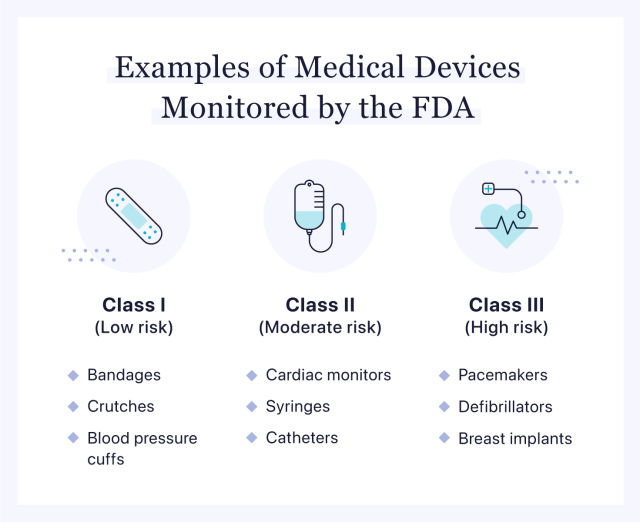
Class I devices are identified as low risk and unlikely to cause bodily harm if a malfunction occurs. These products do not require a rigorous data-focused FDA approval process and account for 47% of the approved medical devices on the market.
Class I devices include bandages, tongue depressors, blood pressure cuffs and crutches.
Class II Devices
Class II devices are considered moderate risk. Although there are more concerns about patient safety with these products than with Class I devices, it is tempered by the presumption that Class II devices are not likely to lead to “preposterous bodily harm” if a malfunction occurs. The FDA reports that 43% of all medical devices are categorized as Class II.
Class II devices include catheters, powered wheelchairs, cardiac monitors and infusion pumps.
Class III Devices
Class III devices are deemed high risk, with the potential to cause serious injury. Because these products are intended to significantly impact patient health, they go through a much more intensive review process before they can be approved for market.
Class III devices include pacemakers, breast implants, mechanical heart valves and defibrillators.
Responsibilities of Manufacturers Under SMDA
Medical device manufacturers are required to submit a report to the FDA when they discover that any of their devices may have caused or contributed to a serious injury or death. If their product malfunctions without causing grave harm but would likely kill or seriously maim a person if the malfunction reoccurred, manufacturers are also required to report it to the FDA.
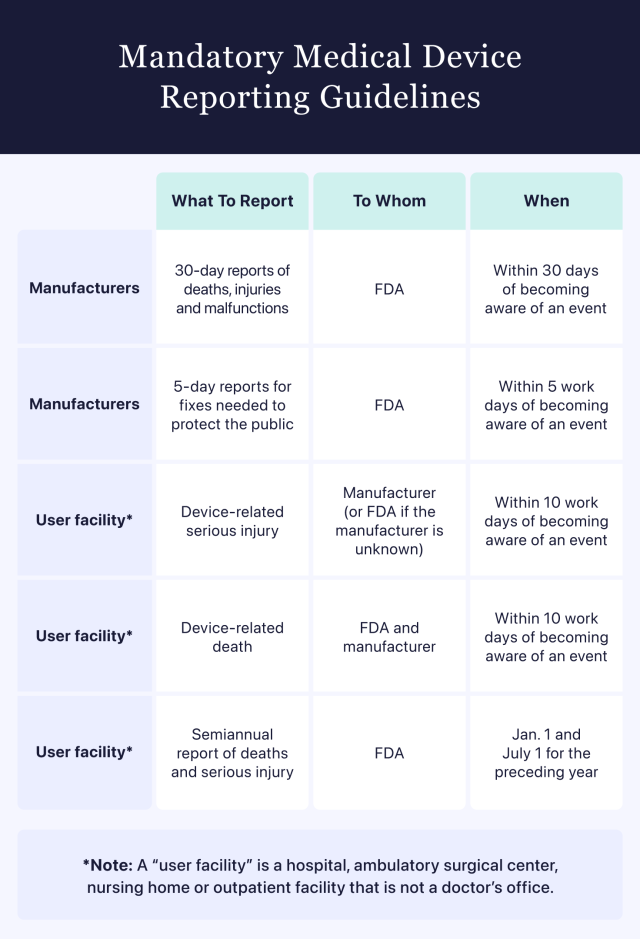
Manufacturers must submit reports tracking deaths, serious injuries and malfunctions that occurred within the previous 30-day period directly to the FDA within 30 calendar days of first learning about the event.
Responsibilities of Health Care Facilities Under SMDA
The FDA refers to a variety of health care operations as “user facilities.” They include hospitals, ambulatory surgical centers, nursing homes and outpatient facilities that are not a doctor’s office. According to SMDA and other FDA guidelines, user facilities are required to report any deaths related to suspected medical device malfunctions. These reports must be sent to both the FDA and the device manufacturer.
User facilities must also report any medical device-related serious injuries. These reports must go to the device manufacturer or directly to the FDA if the manufacturer is unknown.
When reporting a device-related death or device-related serious injury, user facilities must submit the information to the appropriate entity within 10 work days of becoming aware of the event.
Health care professionals at user facilities are also responsible for submitting semiannual reports chronicling deaths and serious injuries to the FDA by Jan. 1 and July 1 of every year.
Advancements in FDA Regulations Since SMDA
The Safe Medical Devices Act of 1990 was a critical step in the FDA’s longtime efforts to monitor the impact of faulty medical devices on public health. But since the SMDA was approved by Congress over 30 years ago, multiple amendments and new regulatory measures have been added to continually improve the efficacy of FDA oversight on medical devices.
The timeline below highlights some of the relevant changes to FDA regulations since SMDA went into effect.
| 1990 | Safe Medical Devices Act (SMDA) | Established a method for monitoring and tracking adverse medical device events |
| 1991 | Manufacturer and User Facility Device Experience (MAUDE) | Adapted the FDA’s medical device reporting system into a searchable online database that catalogs all reported events tied to medical device-related deaths, serious injuries or malfunctions |
| 1996 | Medical Device Reporting (MDR) | Created a reporting system for the FDA, device manufacturers and user facilities to point out and monitor significant adverse events involving medical devices |
| 2002 | Medical Device User Fee and Modernization Act (MDUFMA) | Incorporated user fees for premarket medical device reviews and approved factory inspections to be done by an accredited third party |
| 2018 | Medical Device Safety Action Plan | Developed a unique system for identifying medical devices |
| 2019 | Harmonizing and Modernizing Regulation of Medical Device Quality Systems | Revised existing requirements in an effort to reduce the recordkeeping and compliance burdens on device manufacturers |
Medical devices are an important tool for health care professionals. As the quantity and capabilities of these devices expand, the FDA continues the regulatory journey that began back in 1976. The Safe Medical Devices Act of 1990 laid the foundation for the FDA and consumers at large to have increased access and knowledge about potentially dangerous medical device recalls that can impact millions.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

