Januvia & Janumet
Januvia and Janumet are prescription drugs that belong to a group of Type 2 diabetes drugs called DPP-4 inhibitors. These drugs work by signaling the pancreas to create more insulin and decreasing the sugar made by the liver. However, they can also cause pancreatitis and joint pain.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Patricia Hartke, Pharm.D., BCPS
- Last update: December 1, 2025
- Est. Read Time: 6 min read
Januvia (sitagliptin) is an oral Type 2 diabetes medication manufactured by Merck & Co. The U.S. Food and Drug Administration (FDA) approved the drug in 2006, and it is one of the most popular Type 2 diabetes drugs on the market. In 2007, the FDA approved a variation of Januvia called Janumet, which is a combination of sitagliptin and metformin. Janumet also comes in an extended-release formula called Janumet XR.
Both Januvia and Janumet belong to a class of drugs called dipeptidyl peptidase-4 (DPP-4) inhibitors that work by helping the body produce more insulin. Januvia was the first DPP-4 approved by the FDA.
In clinical trials, Januvia proved effective in controlling blood sugar levels, but some studies reported rare and serious side effects, including acute pancreatitis, severe joint pain and, possibly, pancreatic cancer. However, recent information does not clearly link sitagliptin to pancreatic cancer.
How Do Januvia and Janumet Work?
Sitagliptin, the main active ingredient in Januvia and Janumet, restrains the DPP-4 protein, which plays a role in glucose metabolism. Sitagliptin lowers blood sugar in two ways: It helps the body increase insulin to stabilize blood sugar and decrease sugar made in the liver.
The process works like this: After a person eats, and blood sugar rises, intestinal cells release hormones called incretin hormones. These hormones stimulate pancreatic cells called beta cells to release insulin and metabolize sugar. Additionally, they signal the liver to stop making excess sugar. In people without diabetes, DPP-4 breaks down incretin hormones to balance blood sugar and insulin levels. For people with diabetes, too much sugar is already in the blood.
These drugs block DPP-4, allowing incretin hormones to stay in the blood longer and continue stimulating the pancreas to make more insulin and remove excess sugar.
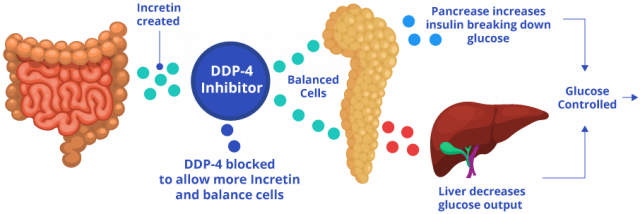
In addition to sitagliptin, Janumet also contains metformin. So, in addition to stimulating the body to produce more insulin like Januvia does, the metformin in Janumet causes the liver to produce less glucose and prevents the intestines from absorbing too much glucose.
How Effective Are Januvia and Janumet?
About 5,200 people participated in the initial clinical trials for Januvia. The majority of study participants were white, and the average age was 55 years. Both Januvia and Janumet reduced blood sugar in clinical trials.
An A1C test measures blood glucose levels. A measurement of 7 percent or lower is ideal for most people with Type 2 diabetes. In one study with 1,262 patients who took Januvia for 18 weeks or 24 weeks, researchers found 100 mg daily lowered A1C by 0.7% and 0.8%, respectively. Januvia also reduced fasting plasma glucose (FPG) by about 7%.
In clinical studies for Janumet, researchers did not actually use Janumet. They obtained their data by administering sitagliptin and metformin. They found that using both drugs lowered A1C and FPG more than using either drug alone. For example, these patients’ A1C levels dropped between 1.4% and 1.9%, compared to a decrease of less than 1% with sitagliptin alone.
Side Effects
In clinical trials, researchers reported the most common side effects that occurred in 5% or more of patients taking sitagliptin include common colds, respiratory tract infections and headaches. Patients may also experience rare but serious side effects.
Patients should consult their doctor immediately if they experience any serious Janumet or Januvia side effects. This is not a complete list of side effects. Always speak to a doctor or health care provider for more information.
- Diarrhea
- Headache
- Nausea
- Sore throat
- Stuffy or runny nose
- Swelling of hands or legs
- Upper respiratory infection
- Allergic reactions (hives, difficulty breathing or swelling of the lips, face, tongue or throat)
- Heart failure (rapid increase in weight, shortness of breath or swelling of the feet)
- Hypoglycemia (low blood sugar)
- Necrotizing pancreatitis (a complication of acute pancreatitis that causes tissue death)
- Acute pancreatitis (inflammation in the pancreas, causing abdominal pain)
- Severe joint pain
- Severe skin reaction (rash, blistering or peeling, burning eyes or facial swelling)
Increased blood glucose, pancreatitis, pancreatic cancer and gastrointestinal disorders (vomiting, diarrhea and nausea) were the most commonly reported side effects of Januvia and Janumet to the FDA’s Adverse Event Reporting System (FAERS).
Other commonly reported Januvia side effects include low blood sugar and headache. Acute kidney injury and lactic acidosis are additional common Janumet side effects listed in FAERS.
| FDA Adverse Events Reporting System (FAERS) Data for Januvia and Janumet Side Effects | Januvia | Janumet |
|---|---|---|
| Total cases reported | 30,280 | 10,289 |
| Serious cases (including deaths) | 15,212 | 6,706 |
| Deaths | 2,713 | 1,335 |
These drugs are not meant to treat people with Type 1 diabetes. People who have excess toxins known as ketones in the blood or urine — a life-threatening condition known as diabetic ketoacidosis — should not take Januvia or Janumet.
Janumet Side Effects and Black Box Warning
Because Janumet is a combination medication, it has the side effect risks of Januvia plus metformin. In people who took Janumet, the most common side effects that occurred in 5% or more of clinical trial participants include diarrhea, headache and respiratory infections. More people who took Janumet instead of Januvia experienced low blood sugar (hypoglycemia) and gastrointestinal issues such as gas, indigestion and abdominal discomfort.
Lactic acidosis is a serious Janumet side effect associated with metformin. This potentially dangerous medical condition occurs when lactic acid builds up in the blood. It causes symptoms such as nausea, vomiting and muscle aches. In more advanced cases, it may cause delirium, loss of balance, low body temperature and low blood pressure.
Lactic acidosis can be fatal if untreated, but stopping metformin may reverse symptoms. Janumet contains a black box warning (the FDA’s most serious medication alert) about the risk of lactic acidosis. The warning explains that symptoms such as fatigue and low blood pressure could indicate lactic acidosis. Additionally, risk factors include kidney issues, being aged 65 or older and excessive alcohol intake.
FDA Warnings and Post-Market Safety Studies
The most serious side effects associated with Januvia and Janumet are acute pancreatitis and severe joint pain.
Between 2006 and 2009, the FDA received 88 reports of acute pancreatitis linked to Januvia and Janumet, some of which were fatal. In March 2013, the agency announced it was gathering more data on Januvia’s link to pancreatitis and possible pancreatic cancer. In 2014, the FDA said it did not appear that sitagliptin drugs cause pancreatitis or pancreatic cancer, but that it had not reached a final conclusion.
In 2015, the FDA warned that Januvia and Janumet could cause severe joint pain. Some patients began feeling this potentially disabling joint pain within just one day of starting DPP-4 treatment, while others developed joint pain years after beginning treatment. Some patients experienced a recurrence of symptoms after restarting DPP-4 treatment.
Drug Interactions
According to the medication inserts of Januvia and Janumet, there are a few drug interactions. But these drugs were not tested with every drug that could react with them.
Patients should use caution when taking Januvia or Janumet with medications known to cause hypoglycemia, or abnormally low blood sugar levels, such as sulfonylureas and insulin.
- Alcohol may increase the risk of lactic acidosis (Janumet)
- Carbonic anhydrase inhibitors such as topiramate, zonisamide, acetazolamide or dichlorphenamide may increase the risk of lactic acidosis (Janumet)
- Digoxin (Januvia)
- Insulin may increase the risk of low blood sugar (Januvia and Janumet)
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




