Lemtrada (Alemtuzumab)
Lemtrada can mitigate multiple sclerosis relapse symptoms, but its long list of side effects has led to black box warnings and other FDA regulations. Before taking Lemtrada, consider the treatment schedule and multiple side effects that surround the drug.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Medically reviewed by Rita Soni, Pharm.D., BCPS
- Last update: November 11, 2025
- Est. Read Time: 14 min read
Lemtrada (alemtuzumab) is a drug that slows the progression of multiple sclerosis (MS) symptoms for relapsing patients. Despite its many side effects, Lemtrada is a popular option for patients who have not had success with other treatments.
Nearly three million people worldwide suffer from MS, with almost one million cases in the U.S. alone. This chronic condition has no known cause and can be debilitating. Studies have found that MS can shorten your life by an average of 7.5 years.
What Is Lemtrada?
Lemtrada is a prescription medication used to treat relapses of multiple sclerosis (MS).
The drug’s manufacturer, Sanofi, claims that 50% of patients do not require additional rounds of Lemtrada or another disease-modifying therapy. Additionally, Lemtrada’s relapse rate is half that of competitor drug, Rebif.
However, there are some serious concerns surrounding Lemtrada. It presents an increased risk for certain autoimmune diseases, cancers and infusion reactions.
Due to these risks, the drug is only available through the Lemtrada Risk Evaluation and Mitigation Strategy (REMS) program. This is a restricted program required by the U.S. Food and Drug Administration (FDA) to make certain that Lemtrada’s potential benefits outweigh its risks.
Key Takeaways
- Lemtrada is a multiple sclerosis drug typically used after other treatments fail. Treatment typically lasts 12 months, with four additional years of health monitoring.
- Available exclusively through the FDA’s Risk Evaluation and Mitigation Strategy (REMS) system, Lemtrada is most commonly administered as an infusion.
- Despite its availability in the U.S. and the European Union, Lemtrada side effects are wide-ranging. The drug has experienced scrutiny from regulatory agencies.
How It Works
Lemtrada targets and eliminates harmful cells created by your immune system. T and B cells are white blood cells that usually protect your body from viruses and bacteria. But if you have MS, these cells can cause nerve damage in your spinal cord and brain.
Lemtrada kills harmful white blood cells, allowing healthier ones to regrow in their place.
Patients typically receive Lemtrada as an infusion, with an initial five-day treatment, followed by a three-day cycle a year later. Many patients do not require further treatment, although some complete one or two more courses.
After completing Lemtrada treatment, patients undergo monthly blood and urine tests, annual skin exams and ongoing symptom self-checks. This lasts for four years or more following treatment.
Common Lemtrada Side Effects
Lemtrada’s label lists over 20 common side effects. Some of the most common problems seen in clinical trial patients include headache, fatigue, rash and fever.
- Arm or leg pain
- Back pain
- Diarrhea
- Dizziness
- Fatigue
- Fever
- Fungal infection
- Headache
- Herpes viral infection
- Hives
- Itching
- Joint pain
- Nausea or vomiting
- Rash
- Sinus infection
- Sore throat or mouth pain
- Stomach pain
- Sudden redness in your chest, face or neck
- Swelling of the nose or throat
- Thyroid issues
- Tingling sensations
- Trouble sleeping
- Upper respiratory infection
- Urinary tract infections
While Lemtrada does not directly cause infections, it may increase the patient’s risk of contracting one. This is why some patients experience side effects like herpes infections.
Serious Lemtrada Side Effects
Lemtrada can cause severe side effects, including infusion reactions, autoimmune issues and elevated cancer risks.
In addition, Lemtrada can increase the likelihood of strokes and torn arteries. Patients have also reported serious adverse reactions in the heart, liver, skin, lymph nodes and thyroid.
- Artery tears
- Blood cancers (lymphoma & lymphoproliferative disorders)
- Infusion reactions
- Severe autoimmune disorders
- Skin cancer (melanoma)
- Stroke
- Thyroid cancer
If you experience an adverse reaction to Lemtrada, seek medical attention immediately.
Fatal Side Effects of Lemtrada Identified in Study
A study in BMC Research Notes used a European database and found nine instances in which Lemtrada was likely linked to fatal side effects and one instance that was possibly related. Most deaths happened within a month of treatment.
- Autoimmune Reactions: Months after treatment, a few patients died from their immune system attacking their bodies. This caused conditions like autoimmune hepatitis and immune-mediated thrombocytopenia (low platelets).
- Infections: Several patients died from infectious diseases like pneumonia and listeriosis within a few weeks.
- Intracerebral Hemorrhage: A woman died from a brain bleed five days after treatment.
- Septic Shock: Some patients experienced severe infections leading to multiple organ failure.
According to the study’s authors, Lemtrada can cause these serious and sometimes fatal side effects in the first month after treatment.
Stroke and Blood Vessel Tears
Some people who received Lemtrada have had strokes or tears in the arteries, known as arterial dissection. These tears may happen in the neck and head within one day of getting Lemtrada. These problems can be hazardous and might cause death or long-term disability.
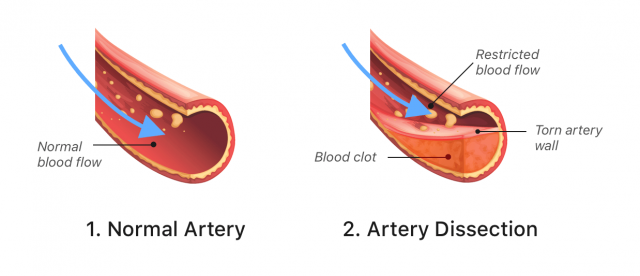
Due to these risks, the FDA added a warning about the dangers of stroke and arterial tear to Lemtrada’s label in 2018.
If you or someone you care for is taking Lemtrada, it’s essential to watch for signs of a stroke.
- Sudden confusion, trouble speaking or understanding speech
- Sudden difficulty walking, dizziness or loss of balance
- Sudden numbness or weakness, especially on one side of the body
- Sudden severe headache or neck pain
- Sudden vision problems in one or both eyes
If any of these symptoms happen, seek emergency medical help immediately. Most of these serious side effects happen within one day of treatment, but they can occur up to three days later. Doctors will closely monitor you after giving you Lemtrada to keep you safe.
Autoimmune Side Effects
Lemtrada can lead to severe autoimmune issues. Autoimmune diseases occur when your immune system mistakenly attacks your body. You may develop conditions where your immune cells attack other organs or tissues in life-threatening ways.
According to Sanofi, Lemtrada can cause rare autoimmune disorders that can cause blood, bleeding, kidney, liver and thyroid problems.
- Acquired hemophilia A (AH)
- Unexplained bleeding in patients with no history of clotting disorders.
- Anti-glomerular basement membrane disease
- A serious condition that, if untreated, can cause severe kidney damage, kidney failure or death. It may require dialysis or a kidney transplant.
- Hepatitis
- Your immune system attacks your liver, causing inflammation. If untreated, this can lead to cirrhosis, liver cancer or liver failure.
- Hemophagocytic lymphohistiocytosis (HLH)
- A rare, life-threatening condition where your immune system attacks your organs and destroys blood cells.
- Hyperthyroidism
- An overactive thyroid.
- Hypothyroidism
- An underactive thyroid.
- Immune thrombocytopenic purpura (ITP)
- Low platelet counts leading to severe, life-threatening bleeding.
Researchers writing in the journal Multiple Sclerosis in 2021 discussed the link between Lemtrada and several autoimmune conditions. According to the authors, these risks were so concerning that in 2019, European health authorities limited their use for individuals with existing autoimmune diseases.
Lemtrada Infusion Reactions
Lemtrada causes infusion reactions for most patients. Around 92% of people experienced this side effect during clinical trials. However, only 3% of these reactions were considered serious.
Severe reactions are rare, but they can be life-threatening and may occur during or up to 24 hours or longer after your infusion.
To lower your risks, Lemtrada is only given in certified health care settings that are prepared to handle emergencies. Health care providers carefully monitor you during the infusion and for at least two hours afterward.
- Chest pain
- Difficulty breathing
- Fast, irregular or slow heartbeat
- Rash
- Swelling in your throat or mouth
- Weakness
Your doctor typically gives corticosteroids before the first three infusions to decrease your likelihood of a reaction. Doctors may also give antihistamines and fever reducers before, during and after treatment. Even with these precautions, reactions can still occur.
Additionally, you will receive antiviral medication to prevent serious infections related to the treatment.
Lemtrada’s Cancer Risk
Lemtrada may increase your risk of some cancers. These include thyroid cancer, skin cancer (melanoma) and blood cancers like lymphoma.
While researchers identified these risks in clinical trials, the overall risk is not much higher than with other treatments. However, caution is still necessary.
These conditions are serious and require immediate medical attention.
Thyroid Cancer
Research has associated Lemtrada with an increased risk of thyroid cancer. In clinical studies, 0.3% of Lemtrada patients developed this type of cancer.
- A new lump or swelling in the neck
- A persistent cough
- Changes in voice, such as hoarseness
- Difficulty breathing or swallowing
- Pain in the front of the neck
You should look for these signs if you are taking Lemtrada. Early detection can significantly improve cancer outcomes.
Melanoma
Patients treated with Lemtrada are at a higher risk of developing melanoma, a severe form of skin cancer. Clinical trials found that 0.3% of Lemtrada patients developed melanoma.
Melanoma often appears as a changing or new mole. It is usually asymmetrical, with irregular borders or color changes. Regular skin checks are essential for catching melanoma early when it is most treatable.
Lymphoma and Other Blood Cancers
Lemtrada is linked to various lymphoproliferative disorders, including lymphoma and rare conditions like Castleman disease and Burkitt’s lymphoma.
These cancers affect your lymphatic system. Symptoms include swollen lymph nodes, unexplained weight loss, night sweats and fatigue.
How Often Do These Side Effects Happen?
Side effects from Lemtrada are wide-ranging, and some reactions occur more frequently than others. Here are some statistics from Lemtrada’s clinical studies:
- When receiving Lemtrada treatment, 92% of patients experienced infusion reactions, with 3% having serious reactions.
- Over 70% of patients developed infections after treatment, with 3% classified as severe cases.
- Almost 37% of Lemtrada patients experienced thyroid disorders, roughly 5% of which were serious.
- Roughly 16% of patients developed herpes viral infections.
- Around 2% of patients developed an autoimmune condition called immune thrombocytopenia (ITP).
- Cervical human papillomavirus (HPV) infection occurred in 2% of patients.
These are just a few reactions patients have experienced. It is essential to share your medical history and prescriptions with your doctor to minimize the likelihood and severity of your Lemtrada side effects.
FAERS and Case Reports
The FDA’s Adverse Event Reporting System (FAERS) is a resource for patients to report adverse drug reactions. It shows that the total number of reported Lemtrada adverse events has decreased over the years, dropping from 1,063 in 2017 to just 158 in 2024.
Of the 5,878 adverse events reported on FAERS for Lemtrada as of November 7, 2025, 4,073 were severe and 199 were fatal. As of November 2025, headache, fatigue and fever were the three most common Lemtrada side effects reported to the FDA.
| Total cases reported | 5,878 |
| Serious cases (including deaths) | 4,073 |
| Deaths | 199 |
It’s important to note that reports sent to the FDA don’t necessarily mean the drug caused an adverse event. Consult with a health care professional before stopping or changing medication.
Lemtrada FDA & EMA Safety Actions
The FDA and its European counterpart, the European Medicines Agency (EMA), have heavily scrutinized Lemtrada. However, their approaches and regulatory actions have differed.
U.S. Regulatory Actions
Before it gained approval for MS treatment under the name Lemtrada, the same drug (alemtuzumab) received its original approval from the FDA in 2001 under the name Campath. At the time, it was used to treat a form of leukemia.
In December 2013, the FDA did not approve Lemtrada’s initial application as an MS drug due to issues with Sanofi’s clinical studies. Lemtrada’s resubmitted application received FDA approval as a treatment for MS relapses in May 2014.
However, the approval came with conditions. Due to its strong side effects, patients can only get a Lemtrada prescription through the FDA’s restricted-access REMS program. The FDA requires this program to ensure that the benefits of Lemtrada outweigh the risks.
The REMS program educates and follows everyone involved in the treatment process. This includes patients, health care providers, pharmacies and facilities. The idea is to carefully monitor patients for potential side effects, including autoimmune conditions and cancer.
The REMS program requires regular tests and check-ups for up to 48 months after the last dose. This can help catch dangerous issues early.
Additionally, before taking Lemtrada, patients should have tried at least two other disease-modifying therapies without success.
In 2018, the FDA warned Lemtrada patients of “rare but serious cases of stroke and tears in the lining of the arteries in the head and neck,” which can lead to permanent disability or death. The FDA required Sanofi to adjust its Lemtrada labeling to include warnings about these risks.
In 2025, the FDA submitted a warning letter to Sanofi for “significant deviations” from standard pharmaceutical manufacturing processes. The letter required Sanofi to investigate and determine the cause of deviations and to prevent them from happening in the future.
Other Regulatory Actions
In 2020, Sanofi agreed to pay $11.85 million to resolve allegations of violating the Anti-Kickback Statute of the False Claims Act. The U.S. Attorney’s Office accused Sanofi of paying kickbacks to Medicare patients, giving them an unfair advantage in the market over other drugs.
The complaint also accused Sanofi of using “a supposed charity” called The Assistance Fund (TAF) to fund these payments, “disguised as routine charitable donations.”
EU Risk Controls
After careful analysis, the EMA ruled that Lemtrada’s benefits outweigh its risks. The agency approved the drug for use in the European Union in 2013.
In April 2019, the EMA initiated a safety review of Lemtrada due to multiple reports of immune system, heart and blood issues. In January 2020, the EMA concluded its investigation and allowed Lemtrada to remain on the market under certain conditions.
The EMA’s new guidelines recommended Lemtrada use only in hospital settings. It should only be offered as a first treatment if the patient has experienced two or more relapses within the last year.
It also fine-tuned its restrictions, disallowing use for those with certain autoimmune, heart, blood and circulation disorders.
Warnings, Contraindications & Interactions
To address the sometimes severe and even fatal side effects of Lemtrada, both the FDA and EMA require proper warnings for patients and health care providers.
Boxed Warnings
Lemtrada now carries several black box warnings (the FDA’s most serious alerts) on its label, including risks of cancer, strokes and more.
- Autoimmune Risks
- Lemtrada may cause serious and sometimes fatal autoimmune illnesses. Health care providers should monitor patients’ blood counts and urine cell counts every month until four years after the final dose.
- Cancer Risks
- Lemtrada may increase the risk of several types of cancer, necessitating annual exams.
- Infusion Reactions
- Serious infusion and allergic reactions can occur with Lemtrada treatment. Medical personnel should monitor patients for at least two hours after each Lemtrada infusion.
- Restricted Prescription Access
- In the U.S., doctors can only prescribe Lemtrada through the FDA’s REMS program.
- Risk of Stroke
- Lemtrada patients have experienced serious and life-threatening strokes within three days of treatment.
Who Shouldn’t Take Lemtrada
Sanofi does not recommend Lemtrada for those with clinically isolated syndrome (CIS), a neurological condition that can be a precursor to MS, or for patients with an active infection, including HIV.
- Another autoimmune condition, in addition to MS
- Blood clotting issues
- Progressive MS
- Serious infections
- Specific heart or circulation issues (including angina, stroke and high blood pressure)
Experts also don’t recommend Lemtrada for patients already taking another disease-modifying therapy for MS. Furthermore, those who are trying to get pregnant should not take Lemtrada.
Those taking Lemtrada should be mindful of their diet, as they are more susceptible to Listeria infections. This means avoiding certain foods, including deli meats, unpasteurized dairy products and undercooked meat, poultry and seafood.
Drug and Vaccine Interactions
Lemtrada can cause complications when combined with many other medications. In total, almost 500 drugs have shown interactions with Lemtrada.
Before taking Lemtrada, talk to your medical provider. Together, you can assess your symptoms and review your current medications to determine if Lemtrada is a suitable option.
Lemtrada Lawsuits — What To Know
Given its many side effects, Lemtrada has been the subject of litigation over the years.
People pursued legal action against Sanofi due to Lemtrada’s ability to cause strokes and torn artery linings, especially if these complications occurred before the drug’s 2018 label change.
Law firms around the country have filed cases involving Lemtrada injuries. Most lawsuits occurred before the label change in 2018.
If you suffered side effects as a result of taking Lemtrada, you may still be able to take legal action. Check our vetted resources to find up-to-date info on Lemtrada lawsuits.
Then, contact a product liability lawyer who can review the details of your case. The statute of limitations to file a lawsuit can vary by state, so it’s best to take action promptly if you think you have a case.
If You Received Injuries After Lemtrada Treatment
If you suffered adverse effects after taking Lemtrada, there are several steps you should take, starting with getting medical care.
- Get medical care: See a medical provider to treat any side effects you may experience.
- Document your symptoms and treatment: Record all of your symptoms and details of your treatment, including a timeline of symptoms and your treatment dates. Your medical records should also include any imaging or test results. Keep all documentation you receive, such as lab results and discharge summaries.
- Report your experience: You can file a report with the FDA by calling 1-800-FDA-1088 or visiting www.fda.gov/medwatch.
- Multiple Sclerosis Association of America (MSAA): Patients can benefit from several assistance programs, as well as a toll-free helpline and online forums.
- Multiple Sclerosis Foundation (MS Focus): This foundation provides a variety of resources, including educational materials, grant programs, support groups and events.
- MyMSTeam: With over 300,000 members, this program provides access to over a dozen online communities for patients. There are also educational resources available.
- National Multiple Sclerosis Society: This organization provides access to patient resources, like financial planning support and a tool to find qualified doctors. There are also community forums and member events, allowing you to connect with other patients.
Reducing Risk & Alternative Treatments
There are steps you can take during Lemtrada treatment to minimize your risk of side effects. Talk to your doctor before, during and after treatment so you can address any issues as they arise. You may need to complete additional tests or lab work as treatment progresses.
Sanofi offers Lemtrada patients access to a case manager for support if they feel anxious about treatment or have questions throughout the process.
Alternatives To Discuss With Your Neurologist
There are some alternatives to Lemtrada for multiple sclerosis treatment.
- Try a different disease-modifying therapy: You can try another FDA-approved disease-modifying therapy infusion, such as Briumvi, Ocrevus and Novantrone. Be sure to review the side effects and discuss them with your doctor to determine if these medications are right for you.
- Switch to an injectable: Instead of undergoing infusion treatments, you can try injectable interferon beta medications. Some of these can be administered at home.
- Take a pill: Some MS drugs are available in pill form, including Aubagio, Tecfidera and Vumerity.
Ultimately, the best MS treatment for you depends on several unique factors, including your preexisting conditions, current medications and allergies, genetics, diet, stress level and environment.
FAQs
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.