Lemtrada
Lemtrada is a monoclonal antibody made by Sanofi Genzyme to treat relapsing forms of multiple sclerosis (MS). The Food and Drug Administration (FDA) originally approved the drug’s active ingredient, alemtuzumab, under the brand name Campath in 2001 to treat a type of cancer called B-cell chronic lymphocytic leukemia (B-CLL). In 2014, the FDA approved Lemtrada to treat MS.
- Medically reviewed by Rita Soni, Pharm.D., BCPS
- Last update: March 13, 2025
In clinical trials, Lemtrada was shown to be more effective than Rebif in slowing the disability caused by MS and preventing relapses. According to Sanofi Genzyme’s 2018 financial report, more than 60 countries have approved the drug to treat adults with relapsing forms of MS. Worldwide net sales of the medication in 2018 were roughly $450 million. In 2022, Sanofi reported a decrease of nearly 50% in sales of Lemtrada from the previous year.
Common side effects of Lemtrada include rash, thyroid problems, infections, body pain, tiredness, trouble sleeping, vomiting and temporary reddening of the skin. However, Lemtrada can also cause more serious side effects and the use of the drug has been restricted by the FDA due to these potential side effects. These effects include autoimmune conditions, infusion reactions and cancer.
Doctors can only prescribe the medication to patients who have not had enough of a response to two or more MS drugs. To be able to dispense, administer or receive the drug, doctors, hospitals, pharmacies and patients must be enrolled in a restricted distribution program called the Lemtrada Risk Evaluation and Mitigation Strategy (REMS) Program.
The drug’s label includes a black box warning for the risk of autoimmune conditions, infusion reactions, stroke and cancer. These warnings are the reason the FDA has restricted use of the drug and has required the drug company to start and manage a REMS program.
The FDA warned the drug could also cause tears in the lining of blood vessels in the head and neck, and lawyers are filing Lemtrada lawsuits on behalf of patients who suffered strokes, head or neck arterial dissections, and/or death.
Treating Relapsing Forms of Multiple Sclerosis with Alemtuzumab
Doctors don’t know exactly how Lemtrada works to treat relapsing MS. According to Sanofi Genzyme’s website, researchers think the drug affects two types of immune cells: B cells and T cells.
Doctors believe these immune cells are overactive in people with MS. This causes them to attack the protective outer layer around nerve cells called the myelin sheath. Eventually, this damages the nerves. The damaged nerves can no longer properly carry signals to the brain or other parts of the body.
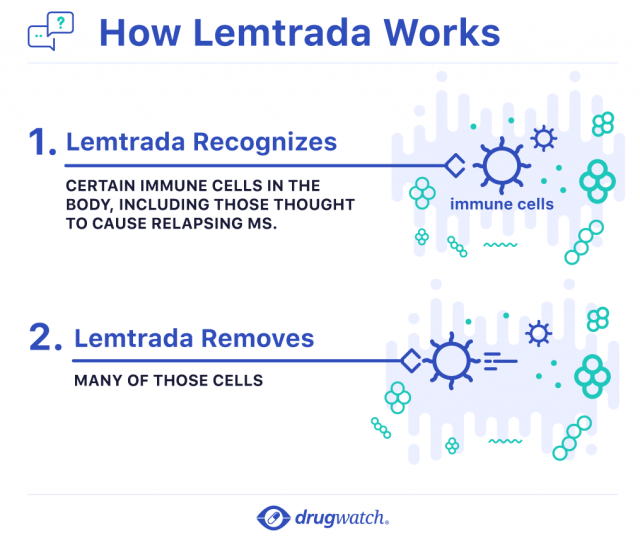
Researchers think Lemtrada targets these B cells and T cells and removes them.
About 30 days after treatment, the drug is undetectable in the body, and the immune cell levels begin to increase over time. After about six months, the B cells levels return to normal, but the T cell levels remain lower than they were before treatment. This effect lasts for at least a year.
What to Expect with Lemtrada Infusions
A doctor’s office, clinic or hospital will administer the drug through intravenous infusion. Because of serious risks associated with the drug, patients cannot administer the medication themselves at home.
Before the infusion, the health care provider will give the patient drugs that will prevent or lessen the effects of infusion reactions. These may include corticosteroids, antihistamines and anti-fever medications called antipyretics.
The infusion itself usually takes about four hours but the entire process can take eight hours. Health care providers are required to take baseline lab tests before starting the infusion. During the infusion, health care providers will closely monitor the patient. For at least two hours after infusion, they will continue to watch for signs of infusion reactions.

Common and Serious Infusion Reactions
The most common Lemtrada infusion reactions include fatigue, shortness of breath, dizziness, pain, upset stomach, nausea, congestion of the lungs, chills, difficulty sleeping, temporary reddening of the skin, hives and itching.
Serious reactions include life-threatening allergic reactions, blood pressure changes, swelling, irregular heartbeat, fever, headache, wheezing, chest pain and rash.
Patients should tell their health care provider immediately if they experience discomfort or anything unusual during their infusion.
Providers may slow down the infusion or stop it temporarily if there are signs of a reaction. They may also administer additional medication to prevent reactions. In some cases, doctors may stop the infusion completely.
Due to the risk of infection, patients who start Lemtrada also need to take a medicine to prevent herpes infection. The medicine will start on the first day of each treatment course and continue for at least 2 months after Lemtrada treatment.
Two Courses of Treatment 12 Months Apart
Lemtrada comes in a single-dose 12 mg vial for injection. Patients will undergo two or more rounds of treatment.
During the first course of treatment, patients receive one infusion every day for five days in a row. Then, 12 months later, patients receive a second course consisting of one infusion every day for three days in a row.
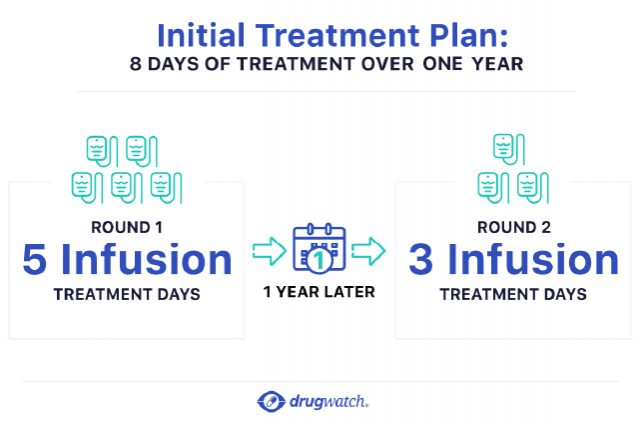
If necessary, 12 months after the second course, patients may receive a third treatment course of 12 mg per day for three days in a row. A health care provider will monitor patients monthly after their first infusion to look for potential side effects. This monthly monitoring will continue for at least four years after the last round of treatment.
Drug Interactions and Precautions
Due to the risk of infection and Lemtrada’s effect on the immune system, patients should receive all required vaccines 6 weeks before starting Lemtrada. The varicella zoster vaccine should also be given 6 weeks before starting Lemtrada to patients who test negative for having antibodies against varicella zoster.
Researchers have not studied how alemtuzumab reacts with other drugs in people with MS. During clinical trials, patients treated with glatiramer acetate and beta interferon had to stop treatment about a month before starting Lemtrada.
Because health care providers administer the drug intravenously, interaction with food and drink are unlikely.
Women of childbearing potential should use contraception during treatment and for four months after treatment because the drug may cause harm to an unborn baby. Researchers did not test the drug in patients younger than 17 years old or in enough patients older than 65 years old.
Patients with HIV should not take Lemtrada.
In clinical trials, two patients had serious reactions after a single accidental infusion up to 60 mg. There is no antidote for an overdose.
Lemtrada Out-Performed Rebif in Two Clinical Trials
Researchers gathered data in two studies involving 1,191 people. The first was a two-year clinical trial comparing Lemtrada and another MS drug called Rebif. People in this trial had a previous MS relapse while on therapy.
The trial found alemtuzumab cut relapses in half over two years. People who took Lemtrada had 49 percent fewer relapses than people who took Rebif. About 65 percent of people on Lemtrada were relapse-free at two years compared to 47 percent of people on Rebif. Lemtrada also out-performed Rebif in slowing disability progression. About 87 percent of people taking Lemtrada did not experience confirmed disability progression versus 79 percent of people taking Rebif.
The second study looked at people who had never taken an MS medication. In this study, alemtuzumab cut relapses by 55 percent and had 78% of patients who were free from relapse at 2 years compared to 59% who received Rebif.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.