Philips Agrees to $1.1B Settlement Over Faulty CPAP Breathing Machines
Editors carefully fact-check all Drugwatch.com content for accuracy and quality.
Drugwatch.com has a stringent fact-checking process. It starts with our strict sourcing guidelines.
We only gather information from credible sources. This includes peer-reviewed medical journals, reputable media outlets, government reports, court records and interviews with qualified experts.
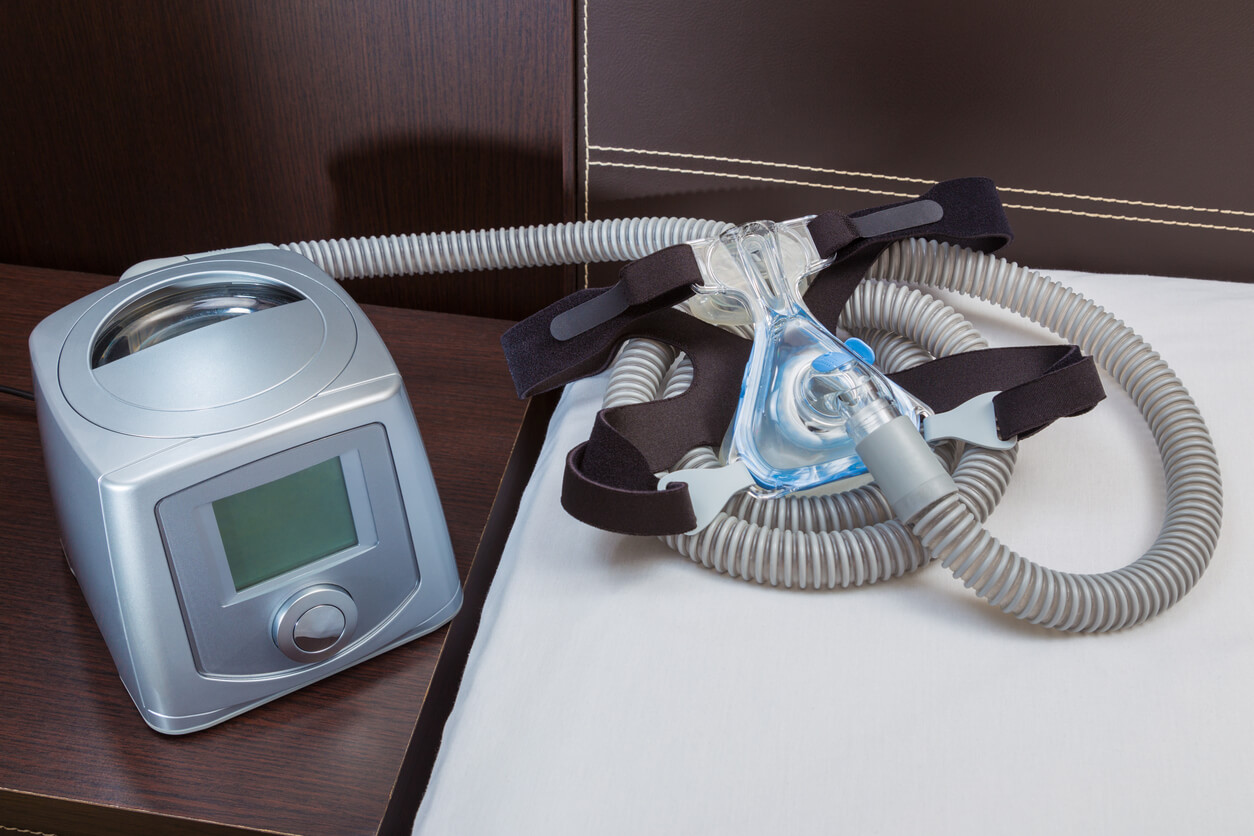
Dutch medical device company Philips Respironics has agreed to a $1.1 billion settlement in the United States to address personal injury lawsuits over its faulty continuous positive airway pressure (CPAP) machines. The new settlement seeks to resolve ongoing legal disputes over Philips DreamStation devices designed to treat sleep apnea.
Reports of adverse health effects, including respiratory conditions and cancer, have caused widespread alarm among Philips CPAP users and their families. The U.S. Food and Drug Administration has received over 105,000 reports of device problems with Philips CPAP and Bi-PAP machines and ventilators — including 385 reported deaths.
Philips CPAP lawsuits claim that foam designed to reduce noise and vibrations in the breathing machines broke down. Patients inhaled or ingested foam particles, leading to serious medical conditions or death, according to the complaints filed in court.
In September 2023, Philips agreed to pay another $479 million to reimburse people who bought, rented or leased any of 20 different models of its breathing machines after the company recalled more than five million devices.
CPAP Settlement Details and Impact
The newly announced settlement includes $1.075 billion for those who suffered personal injury and $25 million for their medical monitoring.
Medical monitoring is intended to address users’ fears of long-term health complications. This settlement would resolve litigation uncertainty for Philips, and company leaders say it highlights the company’s commitment to patient safety.
The agreement is still awaiting final court approval. Still, the announcement is a significant step towards addressing the grievances of thousands of patients who claim Philips failed to warn them about the risks and side effects associated with their CPAP devices.
Philips currently faces 762 CPAP lawsuits in a multidistrict litigation, or MDL, in a Pennsylvania federal court over its CPAP machines used by millions of Americans.
Problems Halted Sales of Philips CPAP Machines in the US
In January 2024, Philips Respironics announced it would stop selling sleep apnea machines and other respiratory devices in the U.S. because of a recall settlement with the federal government.
That decision to halt CPAP sales, which could last for years as the company fulfills a multiyear consent decree with the Department of Justice, marks a significant change for Philips.
According to ProPublica, Philips held a market share of about 37% for sleep apnea devices in the U.S. at the time. The sales halt could have wide-ranging effects on the company and people who need sleep apnea machines.
However, the company has promised continued support for existing machines and will maintain sales outside the U.S. while adhering to the earlier settlement’s terms.