Reglan & Tardive Dyskinesia
Marketed by ANI Pharmaceuticals, Reglan is prescribed for a number of gastrointestinal problems. The drug comes with the risk of several serious side effects, including tardive dyskinesia, a condition involving involuntary facial and body movements. In 2011, Reglan was estimated to be used by more than 2 million Americans.
- Medically reviewed by Kenneth S. Fill, Pharm.D., MBA
- Last update: June 12, 2025
Reglan (metoclopramide) is a prescription medication manufactured by Minnesota-based drugmaker ANI Pharmaceuticals Inc. and is part of a class of drugs called antiemetics. An antiemetic is a drug that is used to treat vomiting and nausea, conditions that can cause vomiting and nausea, or vomiting and nausea caused by certain drug treatments, such as chemotherapy or general anesthetics.
Reglan was approved by the U.S. Food and Drug Administration in 1979, and has been available on the U.S. market as a prescription drug ever since. It is available in orally disintegrating tablets or injections. However, the brand-name oral solution is no longer available. The generic version of Reglan, named after its active ingredient metoclopramide and made by several different manufacturers, is available in both tablet form and oral solution.
Long-term treatment with Reglan may result in adverse reactions including a very serious condition called tardive dyskinesia. TD is a disorder affecting certain muscles in the body, resulting in involuntary, repetitive body movements that may include grimacing, sticking out the tongue or smacking the lips. These movements are a result of the worsening ability of the brain to regulate dopamine over time.
Reglan’s association to development of TD with long-term use necessitated the inclusion of a boxed warning, also called a black box warning, to the drug’s label in 2009. A black box warning is the FDA’s most serious type of warning and is used to call attention to serious or life-threatening risks linked to prescription drug-use.
What Does Reglan Treat?
Reglan treats conditions affecting the gastrointestinal tract including gastroesophageal reflux (also commonly called GERD) and diabetic gastroparesis. Reglan is prescribed for short-term use in patients with GERD who fail to respond to more conventional therapies. The drug can also provide relief of symptoms to patients with acute (sudden and severe) and recurrent (occurring often or repeatedly) diabetic gastroparesis, a condition that occurs when the stomach takes too long to empty food.
Reglan has also been found to be effective in treating other conditions involving nausea and vomiting as well as nausea and vomiting associated with the use of certain medications.
Reglan treats:
- Slow stomach emptying in people with diabetes, also known as gastroparesis
- Nausea and vomiting that can happen with cancer chemotherapy
- Nausea and vomiting that may happen after surgery in patients whose doctors do not want them treated with a stomach tube and suction
What Is Gastroparesis?
Gastroparesis is a chronic condition that reduces the stomach’s ability to empty its contents normally. It does not involve a blockage, or stomach or gastrointestinal obstruction, of any kind.
The disorder is sometimes associated with diabetes, a disease in which a patient’s blood glucose (sugar) levels are too high. It can also occur after some surgeries. However, the exact cause of gastroparesis is unknown, but it may be the result of a disruption of nerve signals to the stomach, according to the National Institutes of Health (NIH). Certain medicines may work to block these nerve, signals causing the complication to occur.
It is estimated that approximately 5 million people in the United States are affected by gastroparesis.
- Nausea
- Vomiting
- Bloating (abdominal distention or premature abdominal fullness after meals)
- Abdominal pain
- Heartburn
- Anorexia (eating disorder characterized by self-starvation and excessive weight loss)
- Weight loss without trying
- Hypoglycemia (abnormally low levels of blood sugar) in patients with diabetes
Possible serious complications of gastroparesis can result from ongoing nausea and vomiting or poor blood sugar control in people with diabetes.
- Dehydration
- Electrolyte imbalances
- Malnutrition
- Diabetes complications
What Is GERD?
Gastroesophageal reflux disease, or GERD, is a condition in which the stomach’s contents leak backwards from the stomach into the esophagus. The esophagus is the “tube” that spans from the mouth to the stomach. When food and stomach acids back up into this tube, it becomes irritated and/or damaged, causing heartburn and other symptoms.
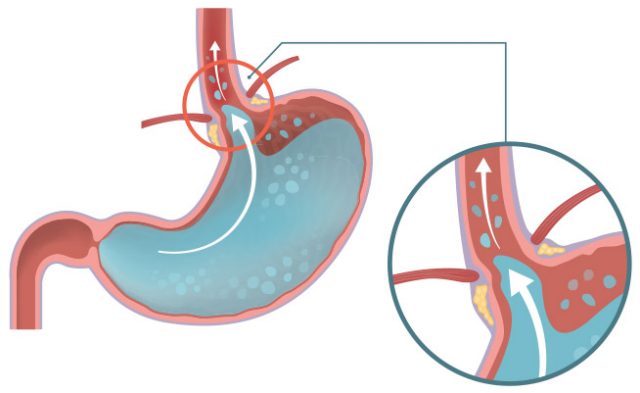
GERD is often referred to as heartburn or acid indigestion. It is a burning sensation in the central part of the chest or upper central abdomen. The pain can also be felt in the neck, throat or jaw area. Other symptoms of GERD might include a feeling that food is stuck behind the breastbone, or nausea after eating.
- Bringing food back up (regurgitation)
- Coughing or wheezing (high-pitched whistling sound made when a person breathes)
- Difficulty swallowing
- Hiccups
- Hoarseness or voice changes
- Sore throat
Symptoms may be worse at night, or when a person bends over or lays down, or after eating.
- Worsening of asthma
- Change in the lining of the esophagus that can increase a patient’s cancer risk (Barrett esophagus)
- Bronchospasm (irritation and spasm of the airways caused by acid)
- Chronic (long-term) cough or hoarseness
- Dental problems due to acid
- Ulcers in the esophagus
- Stricture, or narrowing of the esophagus due to scarring
How Does Reglan Work?
Reglan works in the digestive tract in different ways. For gastroparesis, Reglan works by affecting different receptors in the gastrointestinal (GI) tract, including a dopamine receptor that has a relaxant effect on the gut. Overall, the drug facilitates increased gastric emptying by enhancing contractions in the stomach and small intestine and decreasing relaxation in part of the stomach after meals.
Although Reglan works in most of the GI tract, it has little to no effect on the colon. The drug does, however, increase the tightness of the esophageal sphincter (or muscular ring that closes the esophagus when food is not being swallowed), which prevents the contents of the stomach from coming back up into the esophagus (GERD).
It can also act to relieve nausea and vomiting by blocking dopamine receptors in the brain. Dopamine is a neurotransmitter, or chemical messenger, in the brain that controls the reward and pleasure centers. Thereby, dopamine receptors are able to activate the part of the brain that controls nausea and vomiting. By blocking those receptors, patients may be alleviated of symptoms.
Reglan Side Effects
In approximately 10% of patients given 10 mg of Reglan (metoclopramide) four times daily, the most commonly reported side effects included restlessness, drowsiness, fatigue and lassitude (mental weariness or lack of energy). The incidence of such adverse reactions was dependent upon dosage and duration of drug exposure.
Some adverse reactions may occur after Reglan treatment is discontinued, especially involving the nervous system. These side effects included dizziness, nervousness and headaches.
Use of Reglan, especially for longer than 12 weeks, carries the risk of developing a condition known as tardive dyskinesia (TD), a neurological disorder that involves involuntary, rapid movements of the face and body.
Depending on the person, TD symptoms may be mild and only last for a short time or they may continue indefinitely. If the condition is diagnosed early enough, changing medications may be enough. In some instances, however, it can be permanent.
FDA Requires Black Box Warning for Reglan
According to an FDA study conducted in 2007, a number of patients were prescribed Reglan for long-term use (defined as longer than 3 months) and this increased the number of people who suffered side effects, including TD. The incidence of TD in long-term Reglan users is cited to be as high as 20% in the literature. This led to the FDA requiring a black box warning in 2009. The warning advises patients about the dangers of developing tardive dyskinesia after long-term use of Reglan and its generic counterparts.

The FDA recommends avoiding treatment with Reglan for longer than 12 weeks, but rare cases may arise in which the therapeutic benefit of the drug is deemed to outweigh the risk of TD. Its use should be discontinued in patients who develop signs of TD. In some instances, taking the drug can suppress or partially suppress the signs of TD, masking the underlying disease process.
FDA: Tell Your Doctor About Medical Conditions
If you have kidney problems, your doctor may opt to prescribe a lower dose. For patients with liver problems and heart failure, Reglan may cause the body to retain fluids. And in diabetics, patients taking Reglan may have to have their insulin dose adjusted.
According to the FDA, it is not known if Reglan will harm fetuses. It can pass into breast milk and may harm a breast-feeding infant.
Still, according to some reports, nursing mothers have taken drugs, including Reglan, to help them produce more milk, even though doctors say this can be risky. Lactation consultants have prescribed the drugs, saying they can increase the hormone responsible for breast milk production, prolactin.
- Depression
- Parkinson’s Disease
- High blood pressure
- Kidney problems
- Liver problems
- Heart failure
- Diabetes
- Breast cancer
- Phenylketonuria
- Pregnancy or planned pregnancy
- Breast feeding
Reglan Dosages and Administration
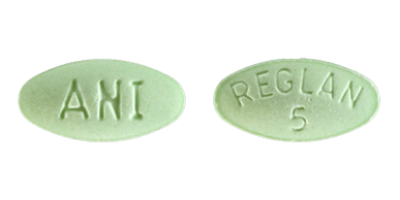
Reglan comes in two tablet strengths – 5 milligrams and 10 milligrams. The pills are differentiated by color and shape as well as the pills’ markings (debossed descriptions).
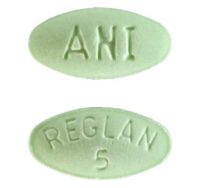
- Green
- Elliptical-shaped (oval shape known as an ellipse)
- Marked with “REGLAN” over “5” on one side and “ANI” on the opposite side

- White
- Double-edge scored
- Capsule-shaped
- Marked with “REGLAN” on one side and “ANI 10” on the other
Gastroesophageal (Acid) Reflux
Patients being treated for gastroesophageal reflux (also more commonly known as acid reflux) should take the recommended dose of Reglan four times a day, either continuously or intermittently (as needed), or at specific times of the day. The recommended adult dosage of Reglan is 10 to 15 mg taken four times daily for four to 12 weeks. Treatment duration is dependent upon the patient’s response to the medication and improvement of their condition as shown by follow-up endoscopic testing.
Reglan works best for acid reflux if taken 30 minutes before each meal and at bedtime. Patients using Reglan to treat acid reflux should not take more than the maximum recommended daily dosage of 60 mg. If taken intermittently, or only as symptoms occur, a single dose of Reglan up to 20 mg can be administered as the situation warrants.
Acute and Diabetic Gastroparesis
The 2022 recommended adult dosage of Reglan for the treatment of sudden and severe diabetic gastroparesis, or gastroparesis that occurs often or repeatedly, is 10 mg taken four times a day for two to eight weeks, depending on a patient’s symptomatic response. Reglan dosages should be taken 30 minutes before each meal and at bedtime. Patients with gastroparesis should not take more than the maximum recommended daily dosage of 40 mg of Reglan.
If patients with diabetic gastroparesis suffer from severe nausea or vomiting and are unable to take Reglan tablets orally, treatment with Reglan can begin by injection. These injections can be given intramuscularly (administered directly into the muscle) or intravenously for up to 10 days. After patients are able to take Reglan orally, treatment should continue with Reglan tablets for no longer than 12 weeks of total treatment time.
Dosage Adjustments and Elderly Patients
Dosage adjustments should be made for patients with the following conditions:
- Moderate to severe liver impairment
- Moderate to severe kidney impairment
- End-stage kidney disease
- Those who poorly metabolize CYP2D6 (an enzyme primarily associated with the liver)
- Quinidine
- Heart medication – helps the heart beat regularly
- Bupropion
- Used to help people quit smoking
- Fluoxetine
- Selective serotonin reuptake inhibitor – SSRI – used to treat premenstrual dysphoric disorder – PMDD
- Paroxetine
- Used to treat depression, anxiety attacks, OCD, panic attacks, PTSD and PMDD
Elderly patients may be more sensitive to the effects of Reglan. A lower starting dosage of 5 mg taken four times a day is recommended. Depending on the patient’s response and tolerability to the drug, the dosage may be gradually increased to the recommended adult dosage of 10 mg taken four times daily.
Reglan Overdose
There is no specific antidote (reversal agent) for Reglan overdose.
- Drowsiness
- Disorientation
- Extrapyramidal reactions (drug-induced movement disorders)
- Other adverse reactions associated with Reglan-use
- Death
Drug Interactions
Reglan’s effects may be intensified or diminished when used concurrently with other medications. Reglan may also affect the clinical efficacy of certain medications in patients. Using Reglan along with other medications can also lead to an increased risk of certain side effects or unknown adverse reactions. Patients should always inform their doctor of all medications and/or supplements they are currently taking before beginning treatment with Reglan.
Medications that may interact with Reglan include:
- Insulin
- Antipsychotics
- Strong CYP2D6 inhibitors – Quinidine, bupropion, fluoxetine, paroxetine
- Monoamine oxidase inhibitors (MAOIs)
- Central nervous system (CNS) depressants – Alcohol, sedatives, hypnotics, opiates, anxiolytics
- Drugs that impair gastrointestinal motility – Antiperistaltic antidiarrheal drugs, anticholinergic drugs, opiates
- Dopaminergic agonists and other drugs that increase dopamine concentrations – Apomorphine, bromocriptine, cabergoline, levodopa, pramipexole, ropinirole, rotigotine
- Succinylcholine, Mivacurium
- Drugs with absorption altered due to increased gastrointestinal motility – Drugs with decreased absorption include digoxin, atovaquone, posaconazole oral suspension, fosfomycin. Drugs with increased absorption include sirolimus, tacrolimus, cyclosporine.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.