Suboxone Tooth Decay Lawsuit
Suboxone lawsuits claim that this drug approved to treat opioid disorder can cause serious dental problems such as tooth decay, oral infections, cavities and tooth loss. Lawsuits claim manufacturer Indivior failed to properly warn about the risks and plaintiffs are seeking compensation.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Trent B. Miracle, Esquire
- Last update: February 5, 2026
- Est. Read Time: 10 min read
- Multidistrict Litigation
- MDL 3092 is in the U.S. District Court for the Northern District of Ohio. U.S. District Judge J. Philip Calabrese presides over it.
- Latest Update
- The number of Suboxone lawsuits has declined slightly in recent months. This reflects recent dismissals for failures to comply with past court orders. Despite the reductions, lawyers continue to file new claims, and the litigation remains ongoing.
- MDL Status
- As of February 2026, there were 1,853 lawsuits pending in MDL 3092.
Am I Eligible For a Suboxone Lawsuit?
- Have been prescribed Suboxone film for opioid addiction or pain management
- Used prescription Suboxone for at least six months before suffering injuries
- Have had routine dental care before Suboxone usage
- Have never used methamphetamine
If you have had dental problems after using prescription Suboxone film, you may qualify to file a Suboxone tooth decay lawsuit.
Only a licensed lawyer can tell you if you qualify to join a Suboxone lawsuit. We’ve vetted national law firms to provide you with the resources you need to file a case.
Can I Still Apply for a Suboxone Lawsuit?
Whether you can still apply for a Suboxone lawsuit will depend on where you live, with statute of limitations playing a key role in this litigation.
The answer won’t be the same for everyone, as the statutes of limitations are very tight in this litigation. If your diagnosis was more than 10 years ago, you must be able to get your own dental records.
But lawyers are continuing to accept new cases from people who qualify.
The easiest way to find out if there is still time for you to sign up is to reach out to a lawyer who can evaluate the details of your case and let you know if you qualify. We can connect you with one of our trusted legal partners for assistance with this.
| Alabama | Illinois | Michigan | New Mexico | South Carolina | Wisconsin |
| Arizona | Indiana | Minnesota | New York | South Dakota | Wyoming |
| Arkansas | Kentucky | Mississippi | North Carolina | Tennessee | |
| California | Louisiana | Missouri | North Dakota | Texas | |
| Colorado | Maine | Montana | Ohio | Vermont | |
| Connecticut | Maryland | Nebraska | Pennsylvania | Washington | |
| Florida | Massachusetts | New Hampshire | Rhode Island | West Virginia |
Why Are People Filing Suboxone Lawsuits?
People are filing Suboxone tooth decay lawsuits because they developed serious dental problems after using Suboxone Sublingual Film, a brand-name medication used to treat opioid use disorder (OUD) or addiction. It contains the active ingredients buprenorphine and naloxone. Lawsuits claim manufacturer Indivior knew or should have known the risks but failed to warn medical providers and consumers.
“Unfortunately, the delivery system has a very high acidic content. And when you use it — which is typically three times a day — you’ve got to keep it in your mouth for about 10 minutes each time to get the full benefit of the dosage,” Miracle told Drugwatch. He explained that the high acidic content throughout the day can result in tooth breakage, loss, decay, and other serious dental issues.”
Drugwatch has heard from countless people who have shared emotional stories about how dental problems after Suboxone use have affected them. These people have worked hard to fight opioid addiction and get their lives back, only to suffer from the loss of their teeth and self-esteem. Many have had to pay tens of thousands of dollars in dental bills. And worse yet, others can’t afford to fix their teeth.
One Suboxone patient shared her experience with us under the initials D.S. to protect her privacy. She suffered dental problems for years and didn’t know it was related to Suboxone until recent research came out. She felt the manufacturer withheld information about the risks to consumers.
“I was known for my smile, and now I’m having these dental issues [after I used Suboxone]. It went from having a few cavities to my whole mouth is cavities. I lost six teeth, so far,” D.S. told us. “It’s so embarrassing. It takes away from my general happiness, my comfort, and my self-esteem. I don’t go out anymore with my friends. If I’m not working, I’m home.”

Suboxone Lawsuit Updates and Timeline
As of February 2026, there were 1,853 Suboxone tooth decay lawsuits pending in multidistrict litigation (MDL) within the Northern District of Ohio.
So far, there have not been any court-approved settlements announced publicly for Suboxone cases related to dental problems, nor have any trials been scheduled. There is a separate pending antitrust MDL related to Suboxone.
Timeline of Suboxone Lawsuits
-
February 5, 2026
The Suboxone litigation continues to progress. A case management conference was recently held where both sides discussed the discovery process.
-
January 8, 2026
As 2026 gets underway, work on the Suboxone lawsuits continues. A conference was slated for this week to discuss the status of the discovery process for this litigation. There are currently more than 1,800 cases pending in federal court.
-
December 3, 2025
The Suboxone lawsuits are still advancing and working through several key issues. Expect more progress and growth for these lawsuits in 2026 as people continue to file new cases.
-
November 5, 2025
Reflecting some of the dismissals we reported on earlier this month, the number of Suboxone lawsuits in federal court has seen a slight dip over the last few weeks. There are now 1,871 active cases in the MDL, which is six cases fewer than at the start of October. People are still continuing to file new lawsuits.
-
October 9, 2025
The judge overseeing the suboxone lawsuits has dismissed some cases from the MDL for not complying with past court orders. Many cases did survive after some plaintiffs showed that they had valid reasons for being behind, such as a death in the family. But cases without an excuse or with a reasoning that the judge did not find compelling were dismissed.
-
July 15, 2025
The Suboxone lawsuits grouped together in federal court continue to progress, with several agreements being reached on key issues following a hearing this week. Another hearing is set for early September as work continues on these cases.
-
June 12, 2025
The judge has ordered a working session to agree on any outstanding discovery and case management issues on June 17. The purpose of this session is to advance the MDL closer to its first trial. Defendants will provide requested documents in July and August, and plaintiffs' lawyers will review these documents. Both sides will also schedule depositions regarding Suboxone's 2022 label change for dental issues.
-
May 23, 2025
A new case management order has laid out a path for eventually picking bellwether trials. The plan is to narrow the potential selection down to 50 total cases. This will include 15 cases picked by plaintiffs, 15 picked by defendants and 20 that are randomly selected. Eventually, that group of 50 will be narrowed down to 15 in a similar manner, with plaintiffs and defendants each picking five cases and five more being randomly selected. This may all sound fairly complex, but it is a big update for the MDL. There is now a clear path forward for the eventual bellwether trials, which will be key to the outcome of the litigation.
-
May 8, 2025
We continue to closely monitor the progress of these lawsuits, and there are no significant updates to report on from the last few days. The next status conference is set for May 15, so we should get some new information on the progress of these cases soon.
-
May 1, 2025
According to a new update, the number of active cases in the Suboxone MDL has once again held steady at 896.
-
April 30, 2025
The show-cause hearing regarding CVS's failure to turn over pharmacy records has been canceled. It was set to take place next week.
-
April 24, 2025
There are no major litigation updates to report on over the last few days following the most recent status conference. We’re continuing to closely monitor the progress of the Suboxone lawsuits and will report on any developments.
-
April 18, 2025
At this week’s status conference, both parties confirmed that they have identified 500 plaintiffs to be part of the record collection pool, which is a key part of the bellwether trial process. A show-cause hearing has also been scheduled for May over CVS’s “[failure] to comply with numerous requests for pharmacy records.”
-
April 14, 2025
A joint agenda has been proposed for this week’s case management conference, with several key issues in the Suboxone MDL set to be discussed. This includes topics critical to the eventual scheduling of bellwether trials, including plaintiff and defendant discovery, as well as a bellwether protocol. The parties plan to submit language on a trial pool sometime in May. This week’s conference is set for April 17.
-
April 10, 2025
We have reviewed all relevant court documents and reached out to plaintiffs' attorneys, and there are no major litigation updates to report on from the last few days. An uptick in filings in the coming weeks will likely follow the judge’s order to allow up to 100 new plaintiffs to be added to the MDL through one complaint.
-
April 7, 2025
The Suboxone MDL continues to progress. In the last week, plaintiffs have filed court documents indicating that they plan to take depositions from Indivior on several topics, including how the company has handled 6 Suboxone complaints, the 2022 Suboxone label change and the corporate structure of the company in relation to Suboxone’s development.
-
April 1, 2025
The number of active Suboxone lawsuits in multidistrict litigation has remained steady, according to newly released statistics. There are currently 896 lawsuits pending in the MDL, which is the same as a month ago. Since the start of 2025, the number of active cases has increased by more than 100.
-
March 14, 2025
In an effort to increase efficiency, the MDL judge overseeing the consolidated Suboxone lawsuits will now allow up to 100 new plaintiffs to join the MDL through a single complaint and filing fee. This should help streamline the process of adding new plaintiffs to the litigation.
-
February 2025
The parties in the Suboxone MDL continue to spar over a path forward, according to newly filed court documents. Plaintiffs say that they have made several concessions to the defendants on how the MDL will move forward, but that the defendants have made little progress on discovery. “The lack of progress on that front portends a lengthy MDL,” the document stated.
-
October 2024
Indivior and other defendants in the MDL are set to produce data on adverse event reports soon. They are expected to be provided before the next status conference, which is set for Nov. 21.
-
September 2024
The MDL may be progressing closer to bellwether trials, with a status conference now set for early October, where forms to collect information as part of the bellwether process will be finalized.
-
June 2024
Judge Calabrese issued an order establishing a procedure to put together a Plaintiffs’ Leadership Development Committee to help less experienced attorneys work with and learn from more experienced lawyers on the Plaintiffs’ Leadership Committee.
According to court documents, lawyers discussed the discovery process and matters related to the statute of limitations tolling agreement. A tolling agreement is an agreement to suspend a statute of limitations (SOL), the time limit to file a claim and protect a person’s ability to file a claim. In this litigation, tight SOLs could bar some people from filing a case within the time limit allowed by their state, especially since it could take a while to get medical records from dentists.
Luckily, in a recent order, the judge allowed plaintiffs’ attorneys to file one complaint with an attachment that lists plaintiffs who face a two-year statute of limitations. This could grow the litigation by several thousand cases overnight.
Judge Calabrese also denied the defendant's request to restrict early discovery in the litigation. They wanted to prioritize discovery into the link between Suboxone and tooth decay. The judge ruled that this would limit scientific evidence and that it would do little to advance the MDL. According to the judge, it would likely "interfere with the search for the truth of general causation." Next, we hope the judge will set some bellwether test trial dates. -
May 2024
Plaintiffs' attorneys submitted proposed deadlines for motions filed by defendants related to preemption of claims and liability depending on when a plaintiff took Suboxone film, according to our research of court documents. Deadlines for responses extend through December 2024.
-
February 2024
The U.S. Judicial Panel on Multidistrict Litigation issued an order to consolidate 15 Suboxone tooth decay claims into an MDL in the Northern District of Ohio. Finally, we have an MDL, and this may help streamline litigation. Our legal partners estimated that there could be hundreds or thousands of people in the litigation.
-
January 2024
The U.S. Judicial Panel on Multidistrict Litigation scheduled a hearing on Jan. 25, 2024, to consider consolidating 15 lawsuits related to Suboxone tooth decay claims.
-
December 2023
The U.S. Judicial Panel on Multidistrict Litigation announced it will consider consolidating 15 Suboxone lawsuits into MDL-3092 during its January 2024 hearing.
-
November 2023
Plaintiffs' lawyers urged the U.S. Judicial Panel on Multidistrict Litigation to centralize all federal Suboxone lawsuits into multidistrict litigation. Meanwhile, 14 new Suboxone tooth decay lawsuits were filed against Indivior in federal courts, with the Northern District of Ohio hosting the highest number of pending cases.
-
October 2023
Indivior agreed to pay $385 million to settle Suboxone lawsuits that drug wholesalers filed.
-
September 2023
David Sorensen filed a Suboxone tooth decay lawsuit against Indivior, Reckitt Benckiser and other defendants after he used the drug and developed permanent damage to his teeth and required substantial dental work.
-
August 2023
Indivior reached a Suboxone settlement for $30 million with health care plans that had brought a federal antitrust lawsuit against the company.
-
April 2023
The Federal Trade Commission announced it had paid about $369,000 to consumers who joined Suboxone class-action lawsuits but missed the original deadline.
-
January 2022
The FDA publicly announced that it received reports that medicines containing buprenorphine that dissolve in the mouth, such as Suboxone, can cause dental problems. Therefore, a new warning was required to be added to the drug’s prescribing information and patient medication guide.
“As warned by the FDA on Jan. 12, 2022, serious dental issues are being reported following use of the sublingual Suboxone delivery system, a strip that contains buprenorphine and dissolves in the mouth. This Suboxone film looks and dissolves much like breath-freshening strips you would buy at a gas station or a grocery store,” Miracle told Drugwatch.
Compensation and Settlements
Based on estimates from lawyers and law firms, a Suboxone lawsuit settlement payout may range from $10,000 to $150,000, depending on the case. Some estimates have been as high as $500,000.
These numbers are speculative, and no global settlement has been announced by plaintiffs or defendants.
- Level of treatment for your injuries (root canals, crowns, dental implants, full-mouth repair and rehab)
- Liens placed on the settlement by your health insurance (to recover payments for your treatment)
- Lost wages resulting from injuries and treatment
- Severity of your injuries
- The location and court where your case is filed or tried
- The strength of your evidence
- Your age
Prior to people filing Suboxone lawsuits for dental problems, Indivior and Reckitt Benckiser faced an antitrust Suboxone class-action lawsuit that claimed limited competition caused consumers to overpay for the drug. In 2019 and 2020, the FTC reached settlements totaling $60 million.
Consumers received the first payments in May 2021, and the remaining class members received payments in April 2023.
The FTC sent about 51,875 class-action settlement payments to consumers. These people allegedly overpaid for Suboxone because of a “deceptive scheme [by Reckitt Benckiser Group and Indivior] to thwart lower-priced generic competition with the branded drug Suboxone.”
Deceptive Marketing Settlement
Indivior has settled other Suboxone lawsuits that were unrelated and separate from the dental injury litigation.
In deceptive marketing claims unrelated to the tooth damage lawsuits, Indivior pleaded guilty to felony charges associated with Suboxone’s deceptive marketing and agreed to pay $600 million to resolve criminal and civil liability in July 2019. Allegedly, the company deceived doctors and health plans, claiming Suboxone was safer and less susceptible to abuse than similar drugs.
Indivior “admitted to an aspect of the scheme alleged in the indictment. Specifically, Indivior Solutions admitted that, in October 2012, it sought to convince MassHealth to expand Medicaid coverage of Suboxone Film in Massachusetts and sent MassHealth false data indicating that Suboxone Film had the lowest rate of accidental pediatric exposure (i.e., children taking medication by accident) of all buprenorphine drugs in Massachusetts, when in fact, it did not,” according to a press release from the U.S. Department of Justice.
The DOJ also reached a $1.4 billion resolution with Reckitt Benckiser, Indivior’s former parent company. Then in October 2019, Reckitt Benckiser reached a $700 million settlement deal with six states for improper marketing.
In October 2023, Indivior settled an antitrust case for $385 million. The settlement resolved claims from direct purchasers of the drug claiming the drug maker illegally tried to monopolize opioid addiction treatment.
Expert and Patient Perspectives
What Is Suboxone and How Does It Work?
Suboxone is a prescription medication approved by the FDA in 2002 to treat opioid use disorder. It combines two active ingredients: buprenorphine (a partial opioid agonist) and naloxone (an opioid antagonist).
It’s available in tablet form or as a dissolvable film that can be placed under the tongue.
The drug works in two ways. Buprenorphine partially binds to receptors in the brain to relieve opioid withdrawal symptoms and reduce opioid cravings without causing a high. Naloxone blocks opioid receptors in the brain to help prevent Suboxone abuse by triggering withdrawal symptoms.
Indivior manufactures brand-name Suboxone, and it’s also available in generic form as buprenorphine and naloxone.
Suboxone Side Effects
The most common side effects of Suboxone include headache, nausea and vomiting. However, Suboxone may have more serious side effects, such as dental problems and life-threatening breathing problems.
Common side effects may be temporary and last for only a few days or weeks. If these don’t go away or bother you, you can talk to your medical provider.
- Headache
- Nausea
- Vomiting
- Constipation
- Pain
- Increased sweating
- Decrease in sleep (insomnia)
Serious Suboxone side effects are rarer when patients take the drug exactly as directed. However, if you experience any of the side effects listed below, you should talk to your doctor right away.
- Trouble breathing
- Don’t take Suboxone with other medicines that contain opioids, such as benzodiazepines, alcohol or other central nervous system depressants. This can cause breathing problems and may lead to coma or death. Seek help right away if you experience dizziness, blurred vision, slurred speech, breathing that’s slower than normal, confusion or trouble thinking clearly.
- Physical dependence or abuse
- It’s possible to abuse Suboxone and become dependent on it, just like other opioids. It can also cause opioid withdrawal symptoms. Call your medical provider if you experience symptoms such as shaking, excessive sweating, diarrhea, muscle aches or vomiting.
- Liver problems
- Before starting Suboxone, your medical provider should do liver function tests. Let your medical provider know right away if you experience yellowing of the skin or eyes, nausea, light-colored stools, pain in the right side of your stomach, loss of appetite or dark urine.
- Allergic reaction
- Allergic reactions can be medical emergencies. Call your doctor or seek emergency help if you experience a rash, face swelling, hives, wheezing or trouble breathing, low blood pressure or loss of consciousness.
- Dental problems
- Suboxone users have reported serious dental problems such as tooth loss or tooth fractures. This can happen in people with no history of poor dental health. Other dental issues include cavities, infections, fillings falling out and total tooth loss.
This isn’t a complete list of all side effects. If you experience any changes in your health or notice new symptoms, make sure you talk to your doctor right away.
Dental Damage and Prevention Tips
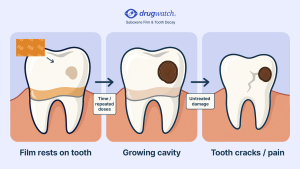 In 2022, the FDA issued a safety communication warning that it had received reports of serious dental problems with Suboxone, including “tooth decay, cavities, oral infections, and loss of teeth.” These problems can be serious and affect people with no history of dental issues.
In 2022, the FDA issued a safety communication warning that it had received reports of serious dental problems with Suboxone, including “tooth decay, cavities, oral infections, and loss of teeth.” These problems can be serious and affect people with no history of dental issues.
Some evidence suggests that Suboxone’s acidity (pH of 3.4) is too high, causing dental problems. High acidity can alter the bacteria on teeth, increasing the risk of cavities. Patients take Suboxone about three times a day, and Suboxone film takes about five to 10 minutes to dissolve. This exposes teeth to the acidic environment multiple times a day for a prolonged period.
- Get regular dental checkups while taking Suboxone
- Gently rinse teeth and gums with water after Suboxone is dissolved
- Wait at least an hour after taking Suboxone before brushing teeth
In addition to the 2022 safety communication, the FDA required Indivior to place a warning on the drug’s label to inform the public about the dental problem reports.
However, Suboxone had been on the market since 2002 without warnings, potentially exposing many patients to tooth damage. Some people who experienced dental problems after using Suboxone films filed lawsuits.
Suboxone Withdrawal and Dependence
While people use Suboxone to treat opioid dependence, it can also be addictive and cause withdrawal symptoms if patients stop taking the drug too quickly.
- Anxiety
- Chills
- Concentration difficulties
- Depression
- Digestive distress
- Drug cravings
- Fever
- Headaches
- Insomnia
- Irritability
- Lethargy
- Muscle aches/body aches
- Nausea
- Vomiting
The severity of symptoms can vary and depends on the strength of the dose and how long a patient has been on the drug. Symptoms may last for as long as a month. Most physical withdrawal symptoms occur within the first 72 hours.
-
72 hours
Physical symptoms
-
1 week
Symptoms include insomnia, mood swings, body aches and pains
-
2 weeks
Psychological symptoms, such as depression
-
1 month
Depression continues, cravings and psychological dependence
To prevent these symptoms, avoid stopping Suboxone abruptly. Be sure to get professional guidance to gradually taper off the medication.
What To Do if You're Experiencing Suboxone Side Effects
If you experience any side effects from Suboxone, make sure you tell your medical provider right away. If you have dental problems, talk to your dentist.
Talk to your medical provider about Suboxone alternatives or if you can taper off the drug safely.
The FDA encourages people to submit reports about side effects to its MedWatch Program.
If you’re considering taking legal action against Indivior for severe dental damage, make sure you get your medical and dental records. Keep any receipts or prescription notes about your history of using Suboxone.
Suboxone Safety in Special Populations
Suboxone isn’t for everyone and has varying levels of safety in pregnant people, children and older adults.
- Pregnancy: Babies born to mothers taking Suboxone may suffer from opioid withdrawal. Symptoms include irritability, hyperactivity, vomiting, diarrhea and failure to gain weight.
- Men and women of reproductive age: Infertility may occur after taking opioids, including Suboxone, and researchers don't know if it's reversible.
- Breastfeeding: Suboxone can pass onto babies through breast milk. Monitor babies for increased sleepiness, drowsiness and trouble breathing.
- Children: Suboxone’s safety hasn’t been tested in pediatric patients.
- Older adults: Suboxone hasn’t been tested on enough adults over age 65 to determine its safety for older people. Seniors should use Suboxone cautiously because of its potential to affect the heart and kidneys.
Before taking Suboxone, make sure you tell your medical provider about any drugs you’re taking and any health conditions you have.
If you or a loved one has experienced severe dental or other complications after taking Suboxone, you may qualify for a lawsuit. Drugwatch can conduct a free, no-obligation evaluation to see if your case qualifies. Reach out today to be connected with a trusted law firm with experience in Suboxone litigation.
How a Lawyer Can Help With Your Suboxone Lawsuit
A lawyer can help you gather the correct evidence of tooth decay or other injuries for your Suboxone case. Your attorney will also file your lawsuit and then negotiate a Suboxone settlement or prepare your case for jury trial.
“As with any case, people should carefully research who they hire to represent them,” Miracle said. “In complex cases such as this, it is especially important to retain counsel with resources and years of experience litigating against multinational pharmaceutical companies.”
Like with most product liability injury claims, lawyers offer free case evaluations for Suboxone teeth lawsuits. Law firms don’t collect their fees unless they recover a settlement or jury verdict on your behalf.
- First Consultation
- During your initial meeting, a lawyer will review your situation, inquire about your Suboxone use and dental injuries, and explain your legal options. This discussion is confidential and typically free of charge, providing you with the opportunity to ask questions about the process and potential outcomes.
- Key Documents To Gather
- To help the lawyer assess your case more efficiently and determine your eligibility for a lawsuit, it's essential to gather your prescription records, dental and medical records, and photographs of your injuries.
- Next Steps After Hiring
- Once you hire a lawyer, they will collect additional evidence and file your claim. Afterwards, they will guide you through the discovery phase, negotiations or trial preparation, depending on the progression of your case.
- Timing Expectations
- Pharmaceutical lawsuits, such as those involving Suboxone, can take months or even years to resolve. It's crucial to file early, as strict deadlines and the case’s complexity can impact how quickly you may see results.
We are investigating product liability cases that claim Suboxone causes tooth decay. You can request a free Suboxone case review through Drugwatch.
Attorney Q&A

Mass torts and product liability attorney Trent B. Miracle, leader of the mass torts division at award-winning law firm Flint Cooper, answered three important questions if you are thinking of filing a Suboxone lawsuit.
- What is the most important thing to know about Suboxone lawsuits?
Time is of the essence if you think you have a claim.
- How does the statute of limitations in certain states affect Suboxone claims?
The statute of limitations likely runs in June 2024 for states with a two-year SOL. But the judge’s recent order will allow us to file a complaint with an attached exhibit listing anyone up against the two-year SOL who chooses to be included.
- What evidence should people have to properly support their Suboxone case?
To evaluate a potential claim, we need to evaluate your pharmaceutical and dental records.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.