Surgical Staplers and Staples
Surgical staplers and staples are medical devices that may be used in place of sutures. They can close large wounds or incisions more quickly and be less painful than stitches for patients. They are often used in minimally invasive surgery. They can also be used to close wounds in areas where skin is tight against bone, in operations to remove organs or to reconnect parts of internal organs.
- Last update: March 7, 2025
Surgical staplers are generally made of plastic and loaded with a disposable cartridge of surgical staples. The staplers come in both reusable and disposable models. They resemble construction or industrial staplers and are designed to insert and close several staples at once.
The devices may be used internally to seal tissue during surgery. They are useful in minimally invasive surgery because they require only a narrow opening and can quickly cut and seal tissue and blood vessels. Skin staplers are used externally to close skin under high tension, such as on the skull or the trunk of the body.
Surgical staples offer several advantages over sutures.
- They can be inserted quickly.
- They’re strong.
- They are easily removed with a surgical staple remover.
- They reduce the amount of time a patient is in surgery and under anesthesia.
When Are Surgical Staplers Used?
Surgical staplers are frequently used to close incisions in the abdomen and uterus during Cesarean deliveries, or C-sections, since the staples allow women to heal faster and reduce scar tissue. Surgeons may also rely on surgical staplers when removing part of an organ or cutting through organs and tissue inside the body.
They are also used to connect or reconnect internal organs within an organ system. The devices are frequently used for surgeries involving the digestive tract, including the esophagus, stomach and intestines, in which a portion of these tube-like structures have been removed and the remaining portions must be reconnected.
Caring for Surgical Staples
Patients must pay special attention to medical staples in the skin to avoid infection. A 2022 study reviewed the surgical site infection rate of wound closure using staples versus sutures in elective knee and hip arthroplasties. The researchers found a significantly higher risk of surgical site infection in patients with staples compared to sutures.
Always follow your doctor’s instructions and do not remove any dressings until it’s safe to do so. Rinse the site twice daily to keep it clean. Your doctor will tell you how and when to dress the wound to prevent infection.
When to Call Your Doctor About Surgical Staple Complications
- Bleeding enough to soak through the bandage
- Brown, green or yellow foul-smelling pus around the incision
- Change in color of the skin around the incision
- Difficulty moving in the area around the incision
- Dryness, darkened skin or other changes around the site
- Fever of 100 degrees or higher for more than 4 hours
- New, severe pain
- Cold, pale or tingling skin near the incision site
- Swelling or redness around the incision
Removing Surgical Staples
Surgical staples usually remain in place for one to two weeks, depending on the type of surgery and the placement of the staples. In some cases, internal staples may not be removed. They are either absorbed or become permanent additions to hold internal tissue together.
Removing surgical staples from the skin is generally not painful. But they should be removed only by a doctor. Never attempt to remove surgical staples on your own.
Removal requires a sterile setting and a specialized surgical staple remover or extractor. The device spreads one staple at a time, allowing the doctor to gently work it out of the skin.
Usually, a doctor will remove every other staple, and a second appointment is scheduled to remove the rest if the wound has not completely healed.
How Surgical Staplers Work
Surgical staplers work by compressing tissue, connecting two pieces of tissue with staggered rows of B- shaped surgical staples and, in some models, cutting away excess tissue to create a clean closure of the surgical wound.
There are various designs for different types of surgeries, with most categorized as either linear or circular.
When using linear staplers, the surgeon uses the handles at one end to close the “jaws” of the stapler at the other end over the tissue. When the surgeon fires the stapler, a row of staples binds the tissue together and a blade cuts the tissue between the staples. The process seals the open wound to prevent bleeding.
Linear staplers are used to connect tissue during minimally invasive surgeries or to remove an organ. Circular staplers are often used for surgeries involving the digestive tract from the throat to the colon.
Circular staplers fire two staggered rows of staples from a circular cartridge. This circular layout allows the stapler to connect two sections of the intestine, or another tube-like structure, after a portion has been removed. The staples cause tissue to pinch up as rings or donuts between the staples. A built-in blade then slices off the overlaying tissue, sealing the new connection.
Surgeons watch the closed wound for about 30 seconds to make sure the tissue has been squeezed together properly and confirm that there is no bleeding.
What Are Surgical Staples Made Of?
Common materials for surgical staples include stainless steel and titanium. These are both strong metals that tend to cause few problems for patients in surgical procedures.
But plastic staples are frequently used for people with metal allergies or to reduce scar tissue.
Staples made from plastic or metals don’t dissolve like many sutures, so extra attention must be paid to prevent infection.
Staples made from polylactide-polyglycolide copolymer are designed to be reabsorbed into the body. They are often used in cosmetic surgery because, like plastic staples, they result in less scaring.
Surgical Stapler Manufacturers
Johnson and Johnson’s Ethicon division and Medtronic are the two largest surgical stapler manufacturers. Together, they produced about 80 percent of the stapler market in 2015, according to an analysis by Future Market Insights. 3M also manufacturers skin staplers sold in the United States.
The devices accounted for close to $2 billion in revenue for manufacturers in 2016, with most sold in North America.
- Ethicon
- Echelon series, Contour Curved Cutter, Endo-Surgery series, Proximate series
- Medtronic
- Signia Stapling System, Endo GIA series of staplers, iDrive Ultra Powered Stapling System, DST series, Premium Plus CEEA Staplers, Appose Single Use Skin Stapler, DFS Single Use Fascia Stapler, Roticulator series, DST Single Use series, ILA series, GIA Single Use and Reusable series
Surgical Stapler Recalls and Injuries
Johnson & Johnson subsidiary Ethicon recalled 92,496 surgical staplers in April 2019 over concerns that they might not fire with enough force to completely form staples.
The U.S. Food and Drug Administration branded the recall as a Class I recall, the FDA’s most serious type. The agency warned in a statement that the devices could cause serious injuries or death. Some people who have been injured by malfunctioning devices have suffered serious injuries and filed surgical stapler lawsuits.
The recall affected two models of the company’s Endo-Surgery Intraluminal Staplers used in gastrointestinal tract surgeries.
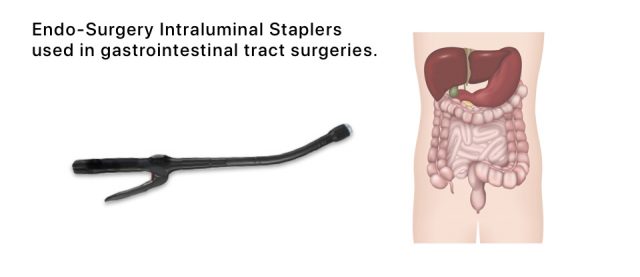
Ethicon reported that two patients had been injured by the devices, according to the FDA. In both cases, the devices misfired, cutting portions of the rectum. Misfires or other malfunctions can prolong operations or require doctors to perform unplanned surgery to correct the damage.
The FDA warned that the misfires could increase complications from surgical staplers, including the risk for bleeding, infection, permanent damage to organs.
In 2015, Ethicon recalled 6,744 Endopath Echelon Flex Powered Vascular Staplers with Advanced Placement Tip and White Reloads. The devices were used in gynecologic, urologic, thoracic, pediatric and general minimally invasive surgeries.
The company reported that an inspection had found the surgical staplers’ cartridges may not insert a complete line of staples when fired.
Medtronic issued two recalls of its Endo GIA staplers and staple cartridges from select production lots, or batches, in 2018 and 2019. Both recalls involved possible missing components. The company said the defects could affect staple alignment and lead to serious complications.
At least five people were injured by staplers included in the 2018 recall, according to the company. The 2019 recall involved defects in staple cartridges that were spotted during the manufacturing process. The company reported “no confirmed complaints” about the devices from doctors or patients.
FDA Actions on Surgical Staplers
The U.S. Food and Drug Administration began tightening restrictions and reporting safety concerns over surgical staplers in 2019. It issued new guidance for using the devices to doctors and hospitals, took steps to reclassify certain surgical staplers from low- to moderate-risk devices and reported tens of thousands of previously unknown cases of stapler malfunctions and injuries.
The new classification would require premarket review and clearance of the devices from the FDA before manufacturers could sell them.
The FDA actions followed a series of surgical stapler problems coming to light earlier in 2019. Kaiser Health News reported that more than half of all surgical stapler malfunctions from 2011 through 2018, 56,000 of them, had been reported to a hidden FDA database instead of a database accessible by the public.
The FDA consolidated the two databases so all the reports could be viewed by the public. The total number of reported surgical stapler malfunctions over the eight-year period rose from 41,000 to nearly 110,000.
-
Jan. 1, 2011 to March 31, 2018
The FDA received over 41,000 individual medical device reports to its public database for surgical staplers and staples for internal use. The reports included 366 deaths, more than 9,000 serious injuries and more than 32,000 malfunctions.
-
March 8, 2019
The FDA sent a letter to health care providers expressing concerns about an increasing number of adverse events associated with surgical staplers and staples for internal use.
-
April 11, 2019
Ethicon recalled circular staplers for insufficient firing and failure to completely form staples. The FDA identified the recall as Class I.
-
April 23, 2019
The FDA issued a draft guidance to help manufacturers develop labeling with information about specific risks, limitations and directions for use of the device.
-
April 24, 2019
The FDA issued a proposed order that would reclassify surgical staplers for internal use from Class I (low risk) to Class II (moderate risk) medical devices with special controls.
-
May 30, 2019
The FDA held an open public meeting of the General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee to discuss whether reclassifying surgical staplers for internal use as Class II medical devices would be appropriate. (The advisory panel reportedly recommended switching the devices to a higher-risk classification with additional safety requirements.)
-
May 30, 2019
A Kaiser Health News report revealed that the FDA acknowledged that more than 56,000 additional surgical stapler malfunctions were quietly reported to the agency from 2011 through 2018, bringing the total to 110,000 malfunctions or injuries during that time period.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


