Vioxx
Vioxx was once a popular drug to treat arthritis. But manufacturer Merck & Co. pulled it from the market in 2004 amid safety concerns. Research linked the drug to thousands of fatal heart attacks.
- Medically reviewed by Amanda Gerberich, Pharm.D. BCPS
- Last update: June 12, 2025
Before it was removed from the market in 2004, Vioxx may have hurt hundreds of thousands of patients, killing a third of them, a senior FDA investigator said at the time.
Dr. David Graham, in interviews and Congressional testimony, criticized his own agency’s approval process of the pain reliever.
Graham described the outcome of Vioxx as “a disaster,” one that is “unparalleled in the history of the United States” and that “constituted an unprecedented failure of the nation’s system of drug approval and oversight.”
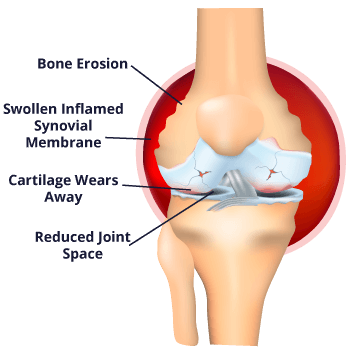
When Vioxx (rofecoxib) received its FDA approval in 1999, it was the second drug of its kind in a relatively new class of painkiller drugs called selective nonsteroidal anti-inflammatory drugs (or NSAIDs). Celebrex (celecoxib) was the first drug in this class.
This new class of NSAIDs, also referred to as COX-2 inhibitors, was claimed to have fewer gastrointestinal (GI) side effects, such as heartburn, nausea, diarrhea, and bleeding in the digestive tract, than older nonselective NSAIDs like ibuprofen.
Vioxx was manufactured by Monsanto and co-marketed by Merck. It quickly became one of the leading agents in providing patients with relief from the painful and bothersome symptoms of various forms of arthritis. It was widely prescribed, used by millions worldwide and brought in billions of dollars in profits for Merck.
Following Vioxx’s success, it was brought to Merck’s attention that selective NSAIDs may offer an additional potential medical benefit. There was evidence that this new class of drugs may contribute to the elimination of colorectal polyps and prevention of colon cancer in individuals likely to develop such conditions. Merck launched a clinical trial called Adenomatous Polyp Prevention on Vioxx (APPROVe) to test the theory.
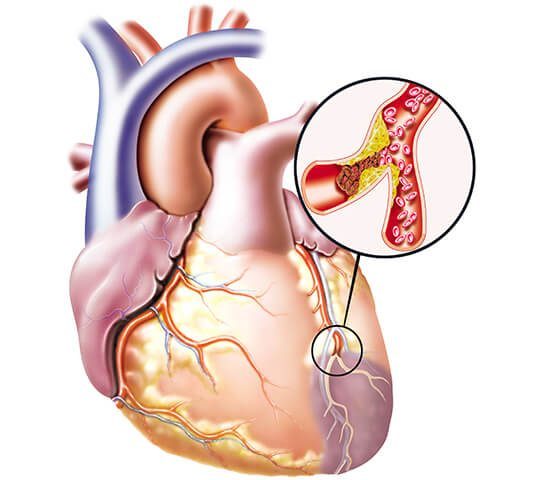
The study revealed bad news for Merck, however. Results showed an increased risk of cardiovascular events, including heart attacks and strokes, in patients taking Vioxx compared to those taking a placebo (sugar pill). This risk was especially apparent for those taking Vioxx for more than 18 months.
Selective Versus Nonselective NSAIDs
Selective NSAIDs, or COX-2 inhibitors, are so named due to their “selectively” in only blocking one type of enzyme directly responsible for pain and inflammation (COX-2). Nonselective NSAIDs, such as aspirin and ibuprofen, relieve pain by blocking the production of two different pain-signaling enzymes, COX-1 and COX-2. The second enzyme (COX-1), not blocked by selective NSAIDs, is thought to help protect the stomach from ulcers.
When blocking COX-2, patients will experience relief from pain felt in joints, muscles and other soft tissues. However, the COX-1 type, which is also blocked by nonselective NSAIDs, plays an important role in protecting the stomach lining. By blocking this type of enzyme, nonselective NSAIDs consequently increase the risk of stomach ulcers and gastrointestinal bleeding.
Selective NSAIDs, however, work to avoid the adverse gastrointestinal effects by only blocking the COX-2 type. This primary benefit set NSAIDs like Vioxx and Celebrex apart from others, and thereby assisted in increased consumer interest and sales.
What Did Vioxx Treat?
Vioxx was a prescription medication used to relieve signs and symptoms of arthritis (joint pain and inflammation), short-term pain in adults and painful menstrual cycles.
FDA – Approved Vioxx Uses:
- Symptoms of osteoarthritis
- Symptoms of rheumatoid arthritis in adults
- Symptoms of pauciarticular or polyarticular course Juvenile Rheumatoid Arthritis (JRA) in patients 2 years and older and who weighed 10 kg (22 lbs.) or more
- Management of acute (short-term) pain in adults
- Treatment of primary dysmenorrhea, or menstrual cramps
- Acute treatment of migraine attacks
Side Effects of Vioxx
Studies in 2022 showed that patients taking Vioxx were at greater risk of heart attack and stroke than those taking older pain reliever alternatives, such as ibuprofen or naproxen, or those not taking any painkillers.
- Respiratory infections
- Headache
- Dizziness
- Diarrhea
- Nausea, vomiting and upset stomach
- Heartburn
- Stomach pain
- Swelling of the legs and/or feet
- High blood pressure
- Back pain
- Tiredness
- Urinary tract infection
Serious side effects:
- Serious stomach problems, such as stomach and intestinal bleeding
- Serious allergic reactions, including swelling of the face, lips, tongue; trouble breathing such as chest tightness or shortness of breath; trouble swallowing; hives; wheezing; or shock (loss of blood pressure and consciousness)
- Heart attacks and other serious cardiovascular events, such as stroke
- Serious kidney problems, including acute (sudden) kidney failure and worsening of chronic kidney failure
- Severe liver problems, including hepatitis, jaundice and liver failure
Number of Patients Affected by Vioxx
At the time of its recall, Vioxx had been taken by some 4 million Americans. Out of those patients who took Vioxx, the arthritis drug may have caused approximately 140,000 heart attacks resulting in an estimated 60,000 deaths, FDA investigator Graham.
Graham concluded that “fundamental problems” within the FDA led to those deaths.
However, another FDA official, Dr. Sandra Kweder, argued against Graham’s calculations pointing out that those estimated deaths “are not real deaths,” but rather based on data, “something you figure out on a spreadsheet.” In other words, Kweder asserted that the deaths Graham referred to were just predictions based on a mathematical model.
Kweder also said Graham’s assessment failed to recognize the benefits of Vioxx, such as it being the only NSAID offering a true gastrointestinal safety benefit. In fact, Merck’s then Chief Executive Officer Ray Gilmartin said he wholeheartedly believed in Vioxx, even having his wife take Vioxx up until the day it was withdrawn from the market.
Heart Attacks Can Happen Within Just Weeks
While the APPROVe trial determined that patients who took Vioxx for longer periods of time, usually 18 months or longer, were at a greater risk of having a heart attack, a newer study showed that some patients likely suffered from a heart attack much sooner after starting treatment with Vioxx.
The newer study was published in the Canadian Medical Association Journal (CMAJ) by McGill University Health Centre (MUHC) in May 2006, nearly two years after Vioxx was voluntarily withdrawn by Merck. The results revealed that a quarter of the patients who had heart attacks while taking Vioxx did so within just two weeks of starting the drug, thereby demonstrating that Vioxx-related cardiovascular risks may occur much earlier than previously thought.
An initial study conducted by university researchers looked at the risk for heart attacks in patients taking COX-2 inhibitors, such as Vioxx and Celebrex. The study concluded that Vioxx carried an increased risk of such cardiovascular events. The newer study then analyzed the pattern of the cardiovascular risk in seniors over a three-year period, thus determining a timeline. This was the first study of its kind to specifically address the timing of cardiovascular risk in patients taking COX-2 inhibitors like Vioxx.
Also, the study found that prolonged use of the drug did not lead to additional cardiovascular risk, suggesting that patients are most susceptible when first starting the medication. Finally, researchers found that a patient’s risk returned to normal within one month of stopping the drug.
-
1999
Vioxx hit the market. The company began distributing the drug to pharmacies across the United States just two days after receiving its stamp of approval from the FDA. Merck needed Vioxx to be successful since four of its existing drugs, bringing in total annual sales of approximately $5 billion, were about to lose their patent protection. This was projected to result in a decline in sales and revenues for Merck as more affordable generic options of its drugs were introduced.
-
2000
In the first four months of 2000, Merck spent $67 million on advertising for Vioxx, which was more than any company had ever spent to advertise any other drug at the time.
-
2001
Vioxx became Merck’s second-biggest drug. However, Merck received lower-than-expected sales results that year with projections of only $3 billion versus an earlier forecast of $3.5 billion. These results were even less promising due to earlier projections of prescription arthritis drugs overall. At the time of Vioxx’s release, analysts projected a surge in sales of prescription arthritis drugs from $7.2 billion worldwide to $13 billion by 2005.
-
2001
Research presented to the FDA in early 2001, provided information regarding potential safety concerns associated with the use of COX-2 inhibitors, specifically Vioxx. The study showed that patients taking Vioxx had a higher, yet still relatively low, risk of heart attack compared to those taking some of the older pain reliever alternatives. Although the risk was seemingly low, with millions taking the drug, the resulting heart attacks could still be quite significant with four heart attacks thought to occur per 1,000 patients. But Merck denied claims that Vioxx increased a person’s risk of heart attack, instead asserting that other pain relievers, such as naproxen, actually decreased a person’s risk of heart attack, resulting in misunderstood findings.
-
2002
Merck agreed to revise its prescribing information for Vioxx to reflect the increased cardiovascular risks associated with the drug compared to older painkiller alternatives, such as naproxen. The decision was made in response to results from the Vioxx Gastrointestinal Outcomes Research (VIGOR) study. In addition to the new warning, however, the FDA allowed Merck to include results from a study on its label describing the benefits associated with the use of Vioxx in reducing certain gastrointestinal side effects, such as ulcers, compared to older pain relievers.
Voluntary Market Withdrawal of Vioxx and FDA Action
Graham, the FDA investigator, released the findings of his critical Vioxx study in August 2004. It found that Vioxx increased the chance of heart attack and death from cardiac arrest significantly more that its number one rival, Celebrex. Furthermore, the study found that dosages of Vioxx in excess of the recommended daily dose of 25 milligrams more than tripled a patient’s risk compared to individuals who had not consumed painkillers within the last two months.
A spokesperson for Merck disagreed with Dr. Graham’s findings, citing that the study would carry more weight if it compared two groups of patients actually taking the medications for a set time period. However, the following month the company made its announcement of a voluntary market withdrawal of the controversial drug, despite Merck’s continued denial of increased cardiovascular risks associated with its leading arthritis painkiller.
On September 30, 2004, Vioxx was pulled from the market, with roughly 2 million people worldwide still using the medication.
FDA Undergoes Congressional Interrogation
Following the voluntary withdrawal of Vioxx, the FDA responded to questions regarding its practices. These questions specifically focused on its expedited review process and its timeliness in conducting and stopping clinical trials when adverse information is found that potentially puts the public at risk.
The FDA was also under criticism for what some described as its seemingly cozy relationship with Merck. At a Senate Finance Committee hearing, witnesses described how danger signals of Vioxx went ignored.
The FDA asserted that Vioxx received a six-month priority review due to the drug’s potential for a significant therapeutic advantage over existing drug alternatives, specifically fewer gastrointestinal side effects such as bleeding. Additionally, while the APPROVe trial enrollment for Vioxx began in 2000, the FDA maintains that it was not stopped earlier because the results for the first 18 months of the trial did not show any increased risk of confirmed cardiovascular events on in patients taking the drug.
However, questions still remain as to the FDA’s dealings with Merck and its handling of Vioxx, and whether it used due diligence in protecting the public from the potentially dangerous effects of the drug. An article written by a professor of medicine and epidemiology and public health, among other academics, and published by the National Institutes of Health, calls attention to Merck’s early suspicion of cardiovascular risk.
The article alleged that even though scientists at Merck had knowledge that the drug might adversely affect the cardiovascular system by increasing thrombus formation, or blood clotting, none of the intervention studies submitted along with its new-drug application to the FDA in 1998 were designed to evaluate such risk. The professors claim that despite FDA concern, Merck was permitted to continue to conceal or misconstrue data in an effort to promote the drug’s cardiovascular safety.
More Stringent Labeling for Selective and Nonselective NSAIDs
While Vioxx is no longer available for sale or purchase by prescription or otherwise, the FDA acknowledged that it did not request the recall of this drug. The FDA did state, however, that it will carefully review any proposal from Merck for renewed marketing of Vioxx and would likely discuss the review with the new FDA Drug Safety Oversight Board and an Advisory Committee before making a final decision.
In any case, the demise of Vioxx resulted in more stringent rules imposed by the FDA on all manufacturers of COX-2 inhibitor drugs and non-selective NSAIDs, both prescription and over-the-counter. The FDA requested that all manufacturers of over-the-counter NSAIDs revise their labeling to include more specific information about potential gastrointestinal and cardiovascular risks, including information to assist consumers in the safe use of such medications.
Additionally, the FDA requested that all manufacturers of all marketed prescription NSAIDs, including Celebrex, revise their labeling for their products to include a black box warning and a medication guide. The boxed warning is required to highlight the potential for increased cardiovascular risks as well as the serious and potentially life-threatening gastrointestinal bleeding that can be associated with such drugs.
In 2015, the FDA released a safety communication about strengthening the current label warning that NSAIDs can increase the risk of heart attack and stroke. Based on newer evidence, the FDA requested that labels be updated to include more information about heart attack and stroke.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

