Synvisc-One Knee Injection Lawsuits
Patients who suffered complications after receiving contaminated Synvisc-One knee injections are filing lawsuits against Sanofi Genzyme and Genzyme Biosurgery. Complaints say patients suffered infections that required medical treatment and/or resulted in death.
- Legally reviewed by Cassandra L. Sundblad, Esquire
- Last update: May 1, 2025
On Dec. 11, 2017, Sanofi Genzyme issued a voluntary recall for 18,000 Synvisc-One knee injection syringes after it had discovered bacterial contamination. More than 12,000 syringes in the lot had been in distribution for up to seven weeks.
Patients injected with the recalled gel reported serious complications, including pain and infections that needed to be treated in emergency rooms and hospitals.
People who required medical attention within a week after receiving a contaminated Synvisc-One knee injection may have a strong case against the manufacturer, Sanofi Genzyme.
Contaminated syringes belonged to lot 7RSL021 and were initially distributed to 36 states on Oct. 25, 2017. People in any of the affected states can file lawsuits in the applicable state’s courts. They may also file lawsuits in federal courts.
People seeking to file a lawsuit may have to prove that they suffered complications after they received a Synvisc-One injection from the recalled lot between Oct. 25, 2017 and Dec. 11, 2017. Though as of November 2022, this litigation has no new updates, and many attorneys have stopped accepting cases.
Injuries from Contaminated Synvisc-One
Synvisc-One injuries are generally related to infections caused by bacterial contamination.
- Infection
- Outpatient procedures (injection, aspiration)
- Emergency room visit
- Hospitalization
- Death
Injured patients may have received an outpatient injection or aspiration with the contaminated product. Others may have received an injection in the emergency room or hospital. Lawyers are also accepting cases on behalf of people whose loved ones died after a Synvisc-One injection.
Settlements and Verdicts for Similar Injuries
People are just beginning to file lawsuits over contaminated Synvisc-One syringes, so there have been no large settlements or large verdicts.
-
May 2014
New England Compounding Center paid $100 million to settle 751 lawsuits after syringes became contaminated with fungal meningitis. The outbreak linked to the syringes was described as the worst of its kind in U.S. history. Among the plaintiffs were the families of 64 patients who died after receiving tainted steroid shots for back pain.
-
May 2016
B. Braun Medical paid $7.8 million to the federal government in penalties and forfeiture and to resolve criminal liability. Pre-filled saline syringes infected patients in four states with Serratia marcescens, a gram-negative bacterium that can cause urinary or respiratory tract infections.
According to the U.S. Department of Justice, B. Braun was aware of problems at the manufacturing plant even before it began purchasing syringes from it. B. Braun approved of changes to the sterilization process even after it received reports of the syringes changing colors. Under the terms of an agreement with the Justice Department, B. Braun said it would increase oversight of its product suppliers by conducting on-site audits of companies that design and make finished products that bear the B. Braun name on the label or logo and testing such products for sterility, identity and purity, as appropriate, on a periodic basis.
AmerisourceBergen Corp. (ABC), one of the nation’s largest wholesale drug companies, agreed to pay $625 million in combined civil and criminal penalties to resolve allegations that it illegally repackaged injectable drugs for cancer patients, according to an announcement from the Justice Department on Oct. 1, 2018. The company was accused of improperly repackaging cancer drugs into prefilled syringes and distributing those syringes to doctors who treated vulnerable cancer patients.
Prosecutors said the company sought to profit from the “overfill” in the original FDA-approved vials by creating a pre-filled syringe program through a repackaging subsidiary that it claimed was a pharmacy. ABC purchased original vials from manufacturers, broke their sterility, combined the contents, and repackaged the drugs under conditions that were not sterile.
FDA Warns Genzyme About Earlier Version of Synvisc-One
In 2001, the FDA warned Genzyme about problems at its Ridgefield, New Jersey, plant. The company made an earlier version of Synvisc at the time.
In its warning, the FDA told the company to fix the problems, including obtaining approval for certain medical products and investigating product complaints, or face severe consequences.
Reports of Complications
The first report of problems with Synvisc-One injections came in less than a month after the contaminated lot shipped.
In November 2017, a medical professional sent a report to the U.S. Food and Drug Administration. It said a patient had developed excess fluid on the knee.
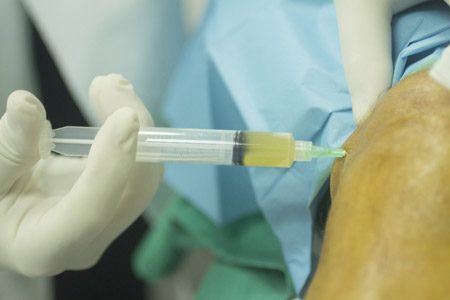
The health care provider had to drain the knee twice. A test of the fluid did not find gram-negative bacteria. But it did find an elevated white blood cell count, an indication that the body is making more white blood cells to fight an infection.
Three weeks later, a woman reported she stumbled trying to stand up after getting an injection. She said her knee could not support her weight.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


