Zofran Lawsuits
Zofran lawsuits claim the drug causes birth defects and that GlaxoSmithKline marketed the drug to pregnant women without FDA approval and failed to warn about risks. A judge ruled the claims were preempted by federal law. Most of the lawsuits were dismissed in 2021, and Drugwatch’s legal partners are not accepting cases.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
I’m so glad this site was invented . It’s helped us so much already . Withou...
- Legally reviewed by Julie Lawson Timmer, Esquire
- Last update: May 1, 2025
- Est. Read Time: 6 min read
- MDL
- U.S. District Court for the District of Massachusetts, MDL No. 2657
- Settlements/Verdicts
- No verdicts or settlements for injury cases
Latest Zofran Lawsuit Updates
As of February 2026, there are no new developments in this litigation. Drugwatch is unaware of any attorneys currently taking Zofran cases.
In June 2021, Judge Dennis Saylor IV of the U.S. District Court for the District of Massachusetts granted summary judgment in favor of GlaxoSmithKline based on federal preemption — i.e., that plaintiffs’ claims about GSK’s labels, which were based on state law, were preempted by federal law, under which the FDA would not have allowed the types of label modifications plaintiffs argued GSK should have made.
The ruling disposed of all 430 cases in the Zofran MDL, including the first bellwether case that had been set for trial in October 2021. In January 2023, the U.S. Court of Appeals for the First Circuit affirmed Judge Saylor’s dismissal, effectively putting an end to the Zofran MDL.
In February 2024, Judge Saylor ruled that plaintiffs must pay GlaxoSmithKline $453,989 in costs ($429,000 of which was related to GSK of obtaining plaintiffs’ medical records), with payment obligations to be allocated equally among the plaintiffs.
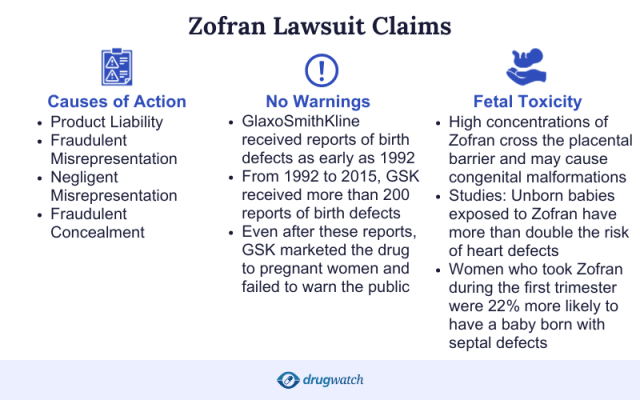
Why People Filed Zofran Lawsuits
Lawsuits against GSK alleged that the company unlawfully and fraudulently promoted Zofran to treat morning sickness, a use not approved by the U.S. Food and Drug Administration. Plaintiffs also alleged the drug caused heart defects, cleft palate and skull deformities in addition to other birth defects.
As a result, GlaxoSmithKline (GSK), the company that originally marketed the drug, was named in more than 600 actions in federal court before the litigation ended in 2021.
They also allege the company misrepresented the results of animal studies, claiming the studies showed the drug was safe — when they actually showed abnormal bone growth and signs of toxicity — and erroneously claimed Zofran was safe for pregnant women.
Zofran Case Study
Flynn v. GlaxoSmithKine LLC
The first Zofran-related lawsuit was filed on Feb. 12, 2015, in the U.S. District Court, Eastern District of Pennsylvania, by Minnesota mother Cheri Flynn on behalf of her two minor children, B.F. and T.F.
Zofran Use:
Flynn was prescribed Zofran early in her first trimester of pregnancy to control morning sickness.
Injuries Claimed:
A daughter born in 2004 was developmentally delayed and had to undergo surgery in 2011 to repair a hole in her heart. A second daughter, born in 2006, also suffered from a congenital heart defect.
Relief Sought:
“Compensatory and punitive damages, equitable relief, and such other relief deemed just and proper arising from the injuries of B.F. and T.F. as a result of their prenatal exposures to the prescription drug Zofran, also known as ondansetron.”
Other Zofran Lawsuit Examples
Zofran users said their babies suffered severe birth defects that render them forever dependent on their parents for care. They demanded compensation for past and future medical bills and cost of care for their children.
- LeClair v. GlaxoSmithKline
- Four days after the first lawsuit was filed, Tomisha LeClair of Massachusetts filed the second. LeClair took Zofran to treat severe nausea and vomiting. Her daughter – identified as A.S. in court documents – was born in 2000 with several congenital defects, including several heart defects, facial dysmorphia, low set ears, hearing loss, webbed toes, sensitivity to light and an inguinal hernia. A.S. had 10 surgeries in 12 years to try to correct several abnormalities.
- Kutzer v. GlaxoSmithKline
- In July 2015, Angela and Brian Kutzer filed a claim that GSK paid doctors to promote and prescribe Zofran and made “false representations about the safety and efficacy” of the drug. The couple’s son was born in 2007 with multiple defects, including a missing kidney and an incomplete vas deferens, the duct through which sperm travels from the testicle to the urethra. The defect may impact his ability to produce children.
- Davis and Hanke v. GlaxoSmithKline
- Ashley Davis and Charles Janke III filed suit in April 2016. Their son, identified as C.J., was born in 2007 with cleft palate and cleft lip after Ashley was prescribed and began taking Zofran early in her first trimester of pregnancy to alleviate morning sickness. The couple says “C.J. has experienced a delay in his physical, language and psychological development and interference with speaking and dental development.”
Lawsuits Moved to an MDL in Boston
In July 2015, GSK asked the Judicial Panel on Multidistrict Litigation (MDL) to centralize the lawsuits in Philadelphia. Plaintiffs in the cases agreed that consolidation was warranted but disagreed on location. The judicial panel issued a court order in October 2015, transferring 12 Zofran lawsuits to the U.S. District Court of Massachusetts.
“Centralization will eliminate duplicative discovery; prevent inconsistent pretrial rulings; and conserve the resources of the parties, their counsel, and the judiciary.”
Judge F. Dennis Saylor IV, who oversaw the cases, appointed three veteran attorneys to lead the litigation against GSK: Kimberly Barone Baden of Motley Rice, Elizabeth Graham of Grant & Eisenhofer and Tobias Millrood of Pogust Braslow & Millrood.
Brief Zofran Litigation History
By December 2015, more than 150 Zofran lawsuits had been centralized in the U.S. District Court of Massachusetts. That month, GSK filed a motion to dismiss the lawsuits. In January 2016, Judge Saylor ruled to allow more than 200 Zofran lawsuits to proceed, rejecting GSK’s arguments for dismissal based on federal preemption — i.e., that plaintiffs’ claims regarding GSK’s warning labels were based on state laws, which are preempted by federal law, under which the FDA would not have permitted GSK to modify the labels the way plaintiffs claimed was appropriate.
Judge Saylor’s rejection of GSK’s argument was based on then-current law, which stated that preemption is a question of fact, making it an inappropriate basis on which to grant summary judgment. Courts may grant summary judgment when there is a question of law, as judges, not juries, are qualified to decide issues of law. When there is a question of fact, however, the jury should decide. However, in May 2019, the U.S. Supreme Court ruled in Merck Sharp & Dohme Corp. v. Albrecht et al that federal preemption questions are questions of law, not fact. Based on that ruling, GSK moved again for summary judgment, and in 2021, Judge Saylor granted the motion based on the new law.
The number of Zofran lawsuits jumped to 238 by March 2016. Two months later, 259 actions had been brought under the MDL.
By September 2018, 623 total actions had been filed and 459 actions were still pending. The discovery phase of the proceedings began in 2016. In October 2018, GSK and plaintiffs selected 16 cases (eight each) for the first bellwether trials. However, the cases never made it to trial, and no settlements were offered before they were all dismissed.
The most recent appeal in these cases was in January 2023, and the appeals court agreed with Judge Saylor’s 2021 ruling that all Zofran cases were preempted by federal law because the FDA would not have allowed the pregnancy warning plaintiffs argued GSK should have made. In March 2024, plaintiffs were ordered to pay GSK $453,989 in litigation costs.
GSK Agreed to Pay $3 Billion For Federal Charges
Prior to the wave of personal injury lawsuits in 2015, the U.S. Department of Justice had brought a lawsuit against GSK, alleging the company “promoted certain forms of Zofran, approved only for post-operative nausea, for the treatment of morning sickness in pregnant women.”
In 2012, GSK pleaded guilty to federal charges of fraud and illegal promotion of several drugs. GSK agreed to pay $3 billion to the U.S. and certain states as part of the legal settlement, which included allegations that the company paid kickbacks to doctors for prescribing Zofran and eight other drugs.
- Knowingly promoted the sale and use of Zofran for a variety of conditions other than those for which its use was approved as safe and effective by the FDA (including hyperemesis and pregnancy-related nausea)
- Made and/or disseminated unsubstantiated and/or false representations or statements about the safety and efficacy of Zofran concerning its use for conditions, including hyperemesis and pregnancy-related nausea
- Offered and paid illegal kickbacks to health care professionals to encourage them to promote and prescribe Zofran, in violation of federal anti-kickback laws
The DOJ described the settlement as “the largest health care fraud settlement in U.S. history.” Then-Deputy Attorney General James M. Cole called it “unprecedented in both size and scope.”
FDA Issued Warning Letter
The Justice Department’s lawsuit was not the first time GSK came under fire for its marketing of Zofran.
In March 1999, the FDA issued GSK a warning letter for distributing promotional materials that present “Zofran in a manner that is false or misleading because it lacks fair balance.”
The agency’s Division of Drug Marketing, Advertising and Communications (DDMAC) concluded promotional material for Zofran violated the Federal Food, Drug and Cosmetic Act because it “fails to present any information relating to the side risks associated with the drug.”
The agency ordered GSK to “immediately cease distribution” of Zofran promotional material that lacks risk information.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.




