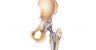
Hip Replacement
A hip replacement, or hip arthroplasty, is surgery to replace a diseased or injured hip joint with an artificial joint or implant. Arthritis-related pain and inflammation, hip fracture and natural wear-and-tear are common reasons for hip replacement surgery.
What Are the Risks and Benefits of Hip Replacement?
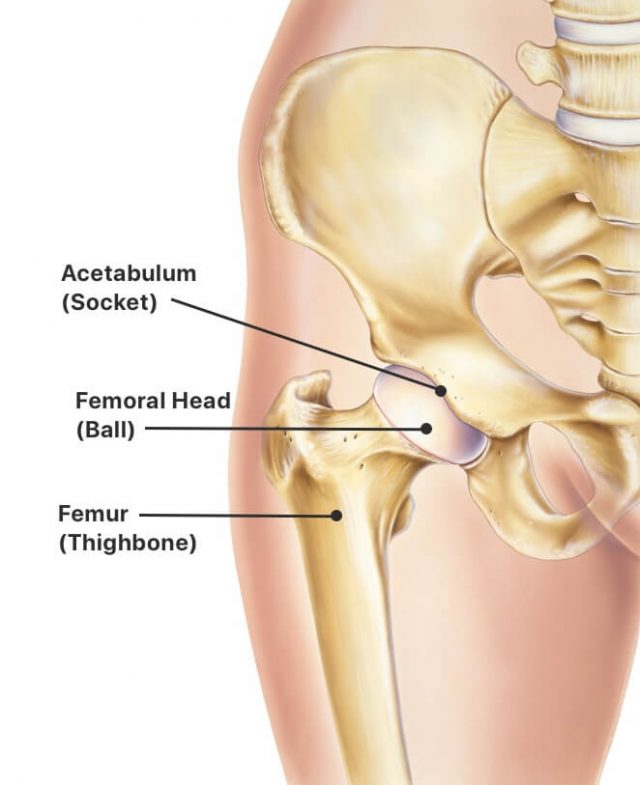
Risks include infection, device failure and surgical complications, but for most patients with severe hip disease, the benefits including eliminated pain and increased mobility greatly outweigh these risks. While common, it is a major surgery that requires weeks to months of recovery.
Some hip replacements require revision surgery to replace worn parts or address complications. According to the report, however, the revision surgery rate has been declining since 2011.
Candidates for Hip Replacement
The most likely candidates for surgery are those in good overall health but experience pain that disrupts regular activities. Doctors may recommend hip replacement for people with osteoarthritis, hip injuries, rheumatoid arthritis, osteonecrosis, bone tumors and limited mobility to help improve overall quality of life.
Be sure to tell your doctor if your consistent pain or lack of mobility causes depression or other changes in your mental health. Talk to your doctor if you experience the following symptoms:
- Consistent pain in your hip or groin even when not moving or standing
- Pain that prevents walking, bending, climbing stairs or daily activities
- Stiffness in your hip that prevents lifting your leg
- Failure of non-surgical treatments to provide relief
Your doctor will rule out other health problems that may cause your pain and mobility issues. Your risk for complications will also be weighed.
Calculating Complication Risks
Dr. H. John Cooper, a board-certified orthopedic surgeon, tells Drugwatch that each individual patient’s age, weight and medical conditions such as diabetes can affect a patient’s risk for complications following hip replacement.
The risk of a specific complication “can be somewhere between 0% and 3% for somebody who’s healthy and in good shape,” Cooper explained. “There are risk factors that are greater than 20% in unhealthy patients with the wrong sort of combination of risk factors.”
Hip replacement patients are typically between the ages of 50 and 80 years old. However, young teenagers with pediatric hip problems including juvenile arthritis and many people over the age of 80, and even 90, have successfully undergone elective hip replacements.
To avoid complications, some doctors may advise avoiding jogging, running or participating in high-impact sports following surgery. They may be able to swim, bike or perform other low-impact activities. Talk to your doctor about their specific recommendations for you.
Alternatives to Hip Replacement
Additional alternative treatments often tried before hip replacement include:
- Exercises that increase muscle around the hip, often under the supervision of a physical therapist
- Canes, walkers and other walking aids
- Over-the-counter medication for pain without inflammation
- Nonsteroidal anti-inflammatory drugs for pain with inflammation
If alternative treatments don’t improve your mobility or quality of life, doctors may recommend a hip replacement procedure. Your medical history, X-rays and physical exams will be reviewed. MRIs or CT scans may also be taken on occasion, although usually are not necessary to make an accurate diagnosis.
Types of Hip Replacement Surgery
Surgeons will often use either a posterior approach or an anterior approach when performing hip replacement surgery. With a posterior hip replacement, the incision is made at the side or back of the hip. During anterior hip replacement, the surgeon makes the incision at the front of the hip.
The posterior approach, which allows better visibility of the hip joint, is more common, but the anterior approach is becoming more prevalent. Many studies show that recovery may be slightly easier and quicker with an anterior approach, but the anterior procedure may pose a higher risk of sensory nerve damage that could cause numbness in the outer thigh.
Special bone cement is sometimes used to hold hip implants in place in patients with weaker bone, but surgeons more commonly use a cementless fixation technique for most patients. Its textured surface allows the bone to grow onto the implant and secure it. A hybrid total hip replacement involves implanting the cup without cement and setting the ball in place with cement.
Total Hip Replacement
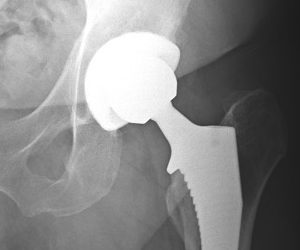
Total hip replacement is the most common hip surgery. It uses artificial components to replace the entire hip structure. During the procedure, surgeons insert a stem into the patient’s femur, or thighbone, for stability. They replace the head of the femur with a ball and replace the natural socket in the hip joint with an artificial cup.
Partial Hip Replacement
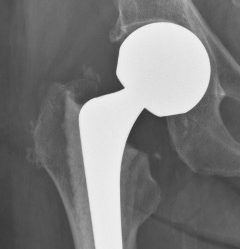
Partial hip replacement removes and replaces the patient’s femoral head, which is the ball at the top of the femur, or thighbone. It does not replace the socket. A ceramic or metal ball is attached to the top of a stem that’s inserted into the hollow center of the femur. Surgeons typically perform this surgery to repair certain types of hip fractures.
Hip Resurfacing
Hip resurfacing helps relieve pain from arthritis in a narrow subset of patients, typically males younger than 55 years of age. A surgeon shaves damaged bone from the natural bone ball at the top of the thigh bone. He or she then resurfaces it with a smooth metal covering. The surgeon also lines the natural bone socket of the hip with a metal lining or shell.
Bilateral Hip Replacement vs. Staged Hip Replacements
Staged hip replacement involves replacing both hips one at a time in two separate surgeries. A bilateral hip replacement involves replacing both hips during one surgery.
A staged hip replacement affords the patient recovery time between the two hip replacements. A bilateral hip replacement means a single trip to the operating room, but isn’t recommended for all patients, particularly not for those who are older or who have certain medical problems.
Because the risk of blood loss during surgery is greater and patients are under anesthesia for twice as long (up to four hours), bilateral hip replacement is typically recommended for younger and healthier patients. Patients are also more likely to require longer hospitalization or inpatient rehab, as well as more rigorous physical therapy and exercise.
Types of Implants
The primary differences between implants are their size and the material of the components. Hip implant components are made of polyethylene (plastic), metal, ceramic or a combination of the materials. All are designed to replicate the body’s natural movements as much as possible.
Metal-on-Polyethylene (MoP)
Metal-on-polyethylene has been used commonly since the 1960s. The ball is made of metal and the socket either has a plastic lining or is made entirely of polyethylene.
Plastic has a smooth surface that causes little friction while the ball moves within the socket. However, MoP implants can produce plastic debris that can eventually cause implant failure and a condition called osteolysis, which occurs when inflammation destroys the bone and the implant in the bone loosens.
While osteolysis was common with older models of hip replacement, most modern designs are markedly better, minimizing the risks of this problem.
Metal-on-Metal (MoM)
Metal-on-metal hip devices are rarely used in the U.S. These hip implants were made from MoM components, meaning the ball, stem and socket were all made out of metal.
The shedding of metal particles could cause a number of serious health issues, including a type of metal poisoning called metallosis. Several models of MoM hips were recalled or taken off the market.
Ceramic-on-Polyethylene (CoP)
Ceramic-on-polyethylene devices couple a ceramic ball with a socket made from plastic. This is, by far, the most common bearing surface used currently in the U.S., as it offers a high rate of clinical success without significant risk. However, there is still a small risk of wear and debris that can cause osteolysis.
Ceramic-on-Ceramic (CoC)
Ceramic-on-ceramic devices combine a ceramic head with a ceramic lining in the hip socket. Studies indicate high success rates of CoC implants. The most common failures unique to this bearing design are squeaking of the bearing surface and ceramic fractures.
Ceramic-on-Metal (CoM)
The U.S. Food and Drug Administration approved the first ceramic-on-metal hip device in 2011. The ball is made of ceramic and the socket has a metal lining.
While used for a short period of time, these are not commonly used today because of risks of metallosis.
Hip Replacement Risks and Complications
Most people who have hip replacement surgery don’t experience major complications. Dislocation, infection and loosening are the most common complications of hip replacement, according to the National Institute of Arthritis and Musculoskeletal and Skin Disease.
An estimated 21.2% of all hip replacements that required revision surgery between 2011 and 2021 were because of infection and inflammatory reactions, according to AAOS’s 2022 report.
Hip surgery complications can include:
- Dislocation
- Loosening of implant
- Infection
- Fracture
- Blood clots
- Bone growth beyond normal edges of the bone
- Leg length inequality
Source: National Institute of Arthritis and Musculoskeletal and Skin Disease
Thankfully, the risks of complications are low. Most modern hip implants can last for decades, even with an active lifestyle.
Hip Implant Recalls
Hip replacement recalls have been issued when implant-caused complications impacted significant numbers of people. Several leading device makers have issued large recalls related to hip complications.
- U.K.-based Smith & Nephew recalled all 6,266 lots of its Modular Redapt Hip Systems in 2016 because of “a higher than anticipated complaint and adverse event trend.”
- Wright Medical recalled shells for its Conserve and Dynasty hip replacement lines in 2016 because of metal debris leading to revision surgeries.
- Smith & Nephew recalled almost 6,000 Birmingham Hip Resurfacing, or BHR, components in 2015 because of “revision rates which were higher than established benchmarks.”
- Stryker recalled 9,003 ABG II Modular hip stems in April 2012 after reports of fretting and corrosion. The junction where the device’s angled necks connect shed particles.
- Johnson & Johnson subsidiary DePuy Orthopaedics issued a global recall for its ASR and ASR XL Acetabular Cups in 2010.
- Zimmer recalled 19,000 Durom cup components in 2008 because of false and misleading labeling.
Some of these recalls are also associated with legal action. More than 20,000 lawsuits over DePuy’s ASR and Pinnacle MoM implants were filed.
What To Know About Metal-on-Metal Implants

“Metal-on-metal implants were commonly used up to a third of the time in hip replacement in the early and mid-2000s,” Cooper told Drugwatch.
The devices had higher than expected failure rates. Today, there are no FDA-approved MoM hip implants available in the U.S. and there are only two FDA-approved MoM resurfacing systems available.
“Often in these patients when inflammation around the joints and local tissue damage develop, if caught early, the bearing surface can be changed so permanent damage is not typically done,” Cooper explained.
In rare cases, if unaddressed, patients can experience serious irreversible, permanent tissue damage or bone loss. In the most extreme cases, patients may experience nerve compression, compression of blood vessels and even side effects related to the heart, vision or hearing.
“The patients with these more problematic or riskier implants need regular follow-up with an orthopedic surgeon familiar with hip replacements,” Cooper said. “If they haven’t been within the past year, I would recommend making an appointment with their surgeon who put the device in.”
Some patients filed lawsuits against hip implant manufacturers, claiming the companies knew their hip systems were defective.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.