Smith & Nephew Hip Replacements
Smith & Nephew makes several hip replacement systems that use patented technology to increase mobility and improve durability. However, the company’s metal-on-metal implants were linked to adverse events. MoM implants are no longer available in the U.S.
Smith & Nephew Expand Hip Replacement Product Offerings
UK-based medical technology company Smith & Nephew recently announced that the first total hip replacement using their new RI.Hip Modeler and the first-ever RI.Hip Navigation procedure on the Cori Surgical System were performed at Duke University in 2022. The RE.Hip Modeler is a preoperative assessment tool and the RI.Hip Navigation is an application that allows for patient-specific component alignment.
In addition to hip replacement technology, Smith & Nephew also manufactures joint replacement systems for knees and shoulders, as well as products used to treat severe wounds, broken bones and sports injuries. The company has expanded its overall orthopedic technology with the 2021 acquisition of Integra’s Extremity Orthopedics business unit for $240 million.
Smith & Nephew reported revenue of $5.212 billion in 2021. It’s annual report noted the company’s market size was 11% of the overall hip and knee implant market, behind Zimmer Biomet with 32%, Stryker with 23% and DePuy Synthes with 20%.
Past Legal Problems
The company paid $28.9 million to the U.S. Department of Justice for illegally paying surgeons to use their implants on patients in 2007. Smith & Nephew was subject to 18 months of federal monitoring as well.
A number of lawsuits were filed against the company following product recalls and reports of complications related to its hip replacement implants. Smith & Nephew warned doctors of the possibility of implant failure because of past use of metal-on-metal components in its devices. MoM implants are no longer available in the U.S.
The metal liner in the R3 Acetabular System was recalled in 2012, for example, because it was associated with implant dislocation, infection and bone fracture that required revision surgery. The Birmingham Hip Modular Head implant was associated with early failure. Smith & Nephew also recalled the Modular SMF and Modular Redapt Revision system in 2016.
Oxinum Oxidized Zirconium
Smith & Nephew describes its proprietary Oxinum Oxidized Zirconium as an “award-winning” implant material that’s beyond a coating, making Oxinium Technology Implants unique. Using a patented heating process, the surface of this metal takes on the wear-resistant properties of ceramic while retaining the strength of the zirconium metal alloy beneath.
Studies have shown that oxidized zirconium improves the durability of joint implants and does not fracture like ceramic. The company says, “The end result is a material that has demonstrated the lowest risk of revision in multiple national and regional hip registries, and the ability to significantly reduce the risk of revision caused by aseptic loosening and infection, versus cobalt chrome implants of the same design in total knee replacement.”
R3 Acetabular System
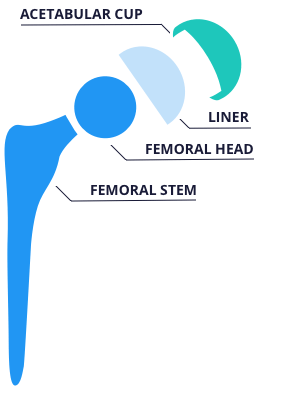
The Smith & Nephew R3 Acetabular System features liners that are designed to sit flush with the rim of the cup and optimized head-to-shell diameter ratios. Bearing options include Oxinium heads on crossed-linked polyethylene, or XLPE, as well as ceramic on ceramic. The company says its Stiktite porous coating is designed for greater cup stability.
What distinguished the R3 System from other hip implant systems when it debuted in the U.S. in 2009 was its ability to be combined with several types of stems, allowing the surgeon to tailor the system to the patient. It was also created with active lifestyles in mind.
The design was intended to enhance range of motion while decreasing the risk of loosening, nerve impingement and dislocation. Its past use of metal liners, however, resulted in patient reports of complications.
R3 Acetabular System and Metal Liner
Many of the problems associated with the R3 Acetabular System were the result of optional metal liners that interacted with metal femoral head components. The release of metal ions and debris into the body, which can cause metallosis or metal poinsoning, were associated with metal-on-metal implants. MoM implants are no longer available in the U.S.
MoM implants were also associated with higher than normal revision surgery rates. Smith & Nephew issued a voluntary recall in 2012. Many patients who received MoM implants and experienced complications filed lawsuits to recover compensation for pain and revision surgery costs.
Emperion Hip System
The Emperion Modular Hip System included titanium alloy stems and sleeves. A reference material entitled “Emperion Surgical Technique” is still available for download on Smith & Nephew’s website, but the Emperion Hip System does not appear in search results for active products.
A study published in January 2016 in Arthroplasty Journal detailed the case of a 67-year-old man whose Emperion hip stem fractured six years after implantation. Another study published in the February 2014 issue of The American Journal of Orthopedics also detailed potential issues with modular hip stems like the Emperion.
The authors of the 2014 study noted that when used with another metal component, such as the Birmingham hip, “crevice and fretting corrosion as well as the potential effect of metal debris generated by MoM articulations” can cause premature fractures and failure.
Modular SMF and Modular Redapt Femoral Hip Systems
Smith & Nephew recalled the modular SMF and Redapt Femoral Hip Systems in 2016. They also recalled the corresponding hip stem components because the company received reports of higher than anticipated complaints and adverse events.
The company analyzed data and found patients implanted with the modular implants may have a higher risk of revision surgery than patients with monolithic products. As with many other metal-on-metal hips, patients with these implants may have adverse reactions to metal debris that may flake off from the junction of the metal neck and stem and embed in surrounding tissues and travel in the bloodstream.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.

