Stryker Hip Replacement Lawsuits
Stryker agreed to a confidential LFIT V40 Femoral Head settlement in 2018. It paid $2 billion in 2014 to settle Rejuvenate and ABG II hip lawsuits. Attorneys are now investigating issues with Stryker Tritanium Acetabular Shells.
Article Continues Below
Drugwatch.com content is legally reviewed for accuracy and quality.
Examples of trusted legal reviewers include qualified mass torts lawyers and certified paralegals.
Drugwatch.com writers gather lawsuit information by studying court records, watching lawsuit proceedings and speaking with experienced attorneys.
Drugwatch.com has been empowering patients for more than a decade
Drugwatch.com has provided reliable, trusted information about medications, medical devices and general health since 2008. We’ve also connected thousands of people injured by drugs and medical devices with top-ranked national law firms to take action against negligent corporations.
Our team includes experienced medical writers, award-winning journalists, researchers and certified medical and legal experts. Drugwatch.com is HONCode (Health On the Net Foundation) certified. This means the high-quality information we provide comes from credible sources, such as peer-reviewed medical journals and expert interviews.
The information on Drugwatch.com has been medically and legally reviewed by more than 30 expert contributors, including doctors, pharmacists, lawyers, patient advocates and other health care professionals. Our writers are members of professional associations, including American Medical Writers Association, American Bar Association, The Alliance of Professional Health Advocates and International Society for Medical Publication Professionals.
About Drugwatch.com
- Assisting patients and their families since 2008.
- Helped more than 12,000 people find legal help.
- A+ rating from the Better Business Bureau.
- 5-star reviewed medical and legal information site.
Testimonials
"Drugwatch opened my eyes to the realities of big pharmacy. Having a family member with major depression and anxiety, I was looking for information on her medications. I found information that was very helpful, that her psychiatrist never told her."
- Defendant
- Stryker
- Injuries in Lawsuits
- Metallosis, loosening, dislocation, pain, revision surgery
- MDL
- MDL 2768 LFIT V40 Femoral Head, MDL 2441 Rejuvenate and ABG II
- Settlements
- $2 billion (Rejuvenate and ABG II implants)
Latest Stryker Hip Replacement Lawsuit Updates
As of October 2024, there were two different multidistrict litigations addressing concerns over Stryker hip replacements, according to data from our review of the most recent MDL Statistics Report.
Each MDL focuses on specific Stryker hip replacement devices, but injuries named in cases are similar and plaintiffs claim they’re the result of design defects within the Stryker models.
MDL number 2768 is before U.S. District Judge Indira Talwani in Massachusetts. There were 209 pending cases as of October 2024 and the MDL addresses LFIT Anatomic CoCR V40 femoral head defects.
MDL number 2441 is before Senior Judge Donovan W. Frank in Minnesota. There were 62 pending cases out of an original 3,637 cases, and the MDL addresses Rejuvenate and ABG II hip implant defects.
Based on our legal research, which included reviewing court documents and talking to our legal partners, we’ve compiled a timeline of key developments in this litigation below.
-
July 2024:
Lawyers continued to take individual cases involving the Tritanium Acetabular Shell. Also this month, a few cases transferred into MDL 2768 from state courts. According to our review of the MDL docket, one of the most recent Stryker LFIT V40 cases was filed by Martin Zakowicz and his wife, Stefanie. The lawsuit, case number 1:24-cv-11251, claimed Zakowicz needed revision surgery because of device defects and excessive levels of cobalt and chromium in his blood.
-
July 2023:
Cases that had been settled continued to be dismissed from MDLs.
-
June 2022:
Judge Frank set a deadline of Sept. 9, 2022, for attorneys to update the Court on any outstanding cases in the confidential Settlement Program in MDL 2441.
-
May 2022:
Several cases were dismissed with prejudice from MDL 2441 because plaintiffs were included in settlement negotiations.
-
June 2021:
Judge Frank postponed discovery in the cases until January 2022 because of the 2020 Settlement Program in MDL 2441.
Even though Stryker offered $2 billion to settle these claims, the MDLs remain open. A few cases continue to transfer into these MDLs, and lawyers are still taking Stryker cases.

Why Are Tritanium Acetabular Shell Lawsuits Being Filed?
People are filing Stryker Tritanium Acetabular Shell hip replacement lawsuits because they claim the devices are defective and shells may loosen and lead to early failure. Loosening may lead to pain, soft tissue damage, bone loss and revision surgery.
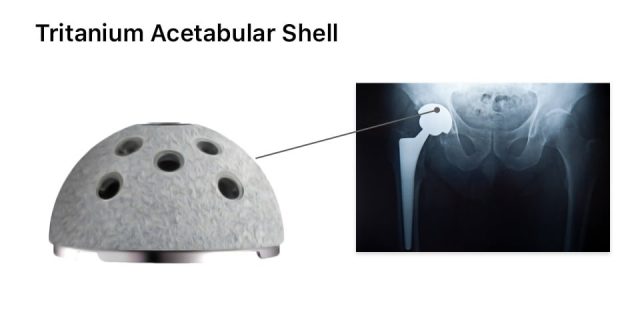
In one of the earliest studies cited in lawsuits, doctors at NYU Langone Orthopedic Hospital provided case studies of five patients who experienced failures of Stryker’s Tritanium cup devices implanted between 2011 and 2016.
These doctors published their findings in Arthroplasty Today. According to the findings, imaging showed loose Tritanium components were damaging the soft tissue surrounding the joint. All five patients underwent revision surgery to correct the issues caused by the devices.
Styker Tritanium Cup Lawsuit Example
Linda Kay Benton v. Howmedica Osteonics Corporation d/b/a Stryker Orthopaedics
Linda Kay Benton filed her lawsuit in New Jersey against Stryker in 2019 after her implant failed in less than four years and she had to have revision surgery.
Tritanium Cup Implantation
Benton received her Tritanium Acetabular Cup in October 2014. Less than four years later, she had to have revision surgery in January 2018.
Injuries Claimed
The lawsuit claimed loosening, discomfort and pain led to her revision surgery. Doctors discovered that the acetabular liner and screws were loose, and the implant didn’t properly fuse with bone.
Relief Sought
Benton sought compensatory damages for physical and mental pain and suffering, medical expenses, lost wages and other damages, as well as punitive damages.
Do You Qualify to File a Stryker Hip Implant Lawsuit?
You may qualify to file a Stryker hip implant lawsuit if you received a Stryker Tritanium Acetabular Cup, LFIT V40, Rejuvenate, ABG II, Accolade, Citation or Meridian implant and suffered complications that led to revision surgery.
- Metallosis, a build-up of tiny metal particles in the blood and surrounding tissues
- Tissue damage or death around the implant
- Implant loosening
- Chronic pain
- Implant failure
- Need for revision surgery
You may also qualify if you need to have revision surgery, but your doctor can’t perform surgery because of potential complications. This is only an overview of potential qualifications, and you should talk to a lawyer about your specific case. Only a lawyer can tell you if you qualify to file a hip lawsuit against Stryker.
Injuries Named in LFIT V40 Femoral Head Lawsuits
Hundreds of people filed LFIT V40 Femoral Head lawsuits after Stryker recalled 42,519 LFIT V40 devices in 2016, citing “higher than expected” complication rates.
One of those claimants was Mern Direnzo, who received the LFIT Anatomic V40 Femoral Head in 2009.
Blood tests showed high levels of metal ions in her blood and urine after the procedure, and Direnzo underwent revision surgery to remove the implant. Direnzo filed a Stryker hip implant lawsuit in 2014.
In November 2018, Stryker and lawyers for plaintiffs suing the company over damages caused by its LFIT V40 Femoral Head device announced an initial settlement agreement. Federal court documents confirm that the agreement included lawsuits combined in a New Jersey multicounty litigation, but the settlement terms are confidential.
Why People Filed Rejuvenate and ABG II Lawsuits
People filed Rejuvenate and ABG II lawsuits after Stryker issued a 2012 Field Safety Notice indicating that its Rejuvenate and ABG II models had a high rate of adverse local tissue reaction caused by inflammation in the tissue near the implant.
The neck components of the Rejuvenate and ABG II models are composed of chromium and cobalt, and the stems have a titanium coating. When the metal parts rub against each other where the neck meets the stem, they can shed metallic debris into the body.
Metal ions released into the surrounding tissue can cause inflammation and prompt an immunological response, leading to metallosis, tissue and bone death, and pain. People with a metal sensitivity may experience a severe allergic reaction.
In many of these cases, patients required revision surgery. In July 2012, Stryker issued a recall for the two models of hip replacement devices. Stryker also offered to reimburse patients for testing, treatment, revision surgery and other expenses connected to hip replacement health complications from the recalled models.
How A Stryker Hip Replacement Lawyer Can Help
A Stryker hip replacement lawyer can help you file your case, fight for you in court and negotiate a settlement on your behalf. The most important qualification to look for in a hip lawsuit attorney is experience with similar hip implant cases.
For example, Drugwatch partners with the award-winning law firm Weitz & Luxenberg. The firm’s lead lawyer on Stryker cases, Ellen Relkin, helped negotiate the last Stryker settlements totaling about $2 billion. She has many years of experience litigating medical device lawsuits.
Stryker hip implant lawsuits are complex litigations that require a legal team with experience handling defective medical device cases. Sometimes, you might not know what type of hip implant you have, and a lawyer can help you find out that information.
“We’re happy to take a look at medical records, take a look at the product itself and see if you had one of those defective products.”
“We’re happy to take a look at medical records, take a look at the product itself and see if you had one of those defective products,” said Daniel Nigh, a mass torts and product liability lawyer.
Ask your potential lawyer about their experience obtaining settlements and jury verdicts against big corporations. An experienced attorney can explain the process, guide you through litigation and help fight for your settlement or jury verdict.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.






