IVC Filter Lawsuits & Verdicts
IVC filter lawsuits allege that C.R. Bard and Cook Medical’s devices had defects that made them more likely to fracture inside or perforate a vein leading to blood clots, organ damage and even death. Bard settled or resolved 8,600 lawsuits. A multidistrict litigation against Cook remains active.
Our content is developed and backed by respected legal, medical and scientific experts. More than 30 contributors, including product liability attorneys and board-certified physicians, have reviewed our website to ensure it’s medically sound and legally accurate.
legal help when you need it most.
Drugwatch has provided people injured by harmful drugs and devices with reliable answers and experienced legal help since 2009. Brought to you by The Wilson Firm LLP, we've pursued justice for more than 20,000 families and secured $324 million in settlements and verdicts against negligent manufacturers.
More than 30 contributors, including mass tort attorneys and board-certified doctors, have reviewed our website and added their unique perspectives to ensure you get the most updated and highest quality information.
Drugwatch.com is AACI-certified as a trusted medical content website and is produced by lawyers, a patient advocate and award-winning journalists whose affiliations include the American Bar Association and the American Medical Writers Association.
About Drugwatch.com
- 15+ Years of Advocacy
- $324 Million Recovered for Clients
- 20,000 Families Helped
- A+ BBB Rating
- 4.9 Stars from Google Reviews
Testimonials
I found Drugwatch to be very helpful with finding the right lawyers. We had the opportunity to share our story as well, so that more people can be aware of NEC. We are forever grateful for them.
- Legally reviewed by Tess Schulman, Ph.D.
- Last update: November 3, 2025
- Est. Read Time: 8 min read
Latest IVC Filter Lawsuit Updates
As of February 2026, there are 6,873 pending cases in multidistrict litigation – MDL 2570 – against Cook Medical before Senior Judge Richard L. Young of the U.S. District Court for the Southern District of Indiana. A total of 11,464 lawsuits have been filed in the MDL. There are also individual lawsuits against IVC filter manufacturers ALN, Argon, Boston Scientific, Cordis and Rex Medical are also in progress.
The inferior vena cava filter MDL against C.R. Bard closed with confidential settlements taking place. Maria Dalbotten’s case was on its way to trial but was dismissed in June 2023.
- October 2025: Court documents show that both sides have “reached agreement on the major terms and conditions of settlement” for some of the cases in the MDL. It’s important to note that this applies to just a group of the active cases. In recent weeks, the judge has also entered judgment in favor of the defendants for several individual cases.
- August 2025: Settlement discussions remain well underway for the Cook IVC filter lawsuits. A series of settlement conferences are currently scheduled for the end of October. As these discussions take place, our partners continue to investigate and accept new cases.
- April 2025: The court has set key deadlines relating to the upcoming settlement conference in the Cook IVC MDL. Over the next few weeks, parties will establish how many pending lawsuits there are for each injury category. Plaintiffs and defendants will then submit competing breakdowns of what settlement value they ascribe to each injury category.
- March 2025: Settlement discussions are underway in the Cook IVC MDL. Both parties met for a status conference on March 20, where steps were taken to begin moving toward a settlement. A settlement conference is set for April 24.
- October 2024: There are no big updates to report from the last few months. The Cook MDL is still active but has been moving at a slow pace. We’re continuing to keep a close eye on its progress.
- May 2024: Cook Medical successfully dismissed a few cases in the MDL. New plaintiffs’ lawyers filed their notice of appearance.
- March 2024: The Arizona MDL for C.R. Bard remains open, though zero cases remain active.
- February 2024: Judge Young set a status hearing for MDL 2570 for June 2024. Cases from state courts across the country continue to transfer into the MDL.
- November 2023: The Court entered summary judgement in Ernie Scott v. Cook et al., so the case will not go to trial.
- June 2023: Pretrial conference in the Cook MDL scheduled for August 2023.
- May 2023: Cook settled some cases in the MDL for confidential amounts, but thousands remained pending. The judge scheduled the next Cook Medical bellwether trial, Scott v. Cook Medical, for Dec. 4, 2023.
- January 2020: The U.S. District Court for the Southern District of Indiana vacated the $3 million judgment against Cook Medical in Brand v. Cook Medical. The court said the plaintiff didn’t have enough evidence.
- May 2019: Bard reached a confidential settlement agreement with about 8,000 plaintiffs.
- February 2019: A jury awarded $3 million to plaintiff Tonya Brand in Brand v. Cook Medical.
Unlike Bard, Cook Medical has yet to reach any notable or global settlements. In November 2017, Cook Medical won the first bellwether trial in its MDL. However, it lost the next two. Juries awarded $1.2 million and $3 million to the plaintiffs.
Legal and Personal Perspectives on IVC Filter Injuries
Why Are People Filing IVC Filter Lawsuits?
People who had an inferior vena cava filter implanted are filing lawsuits after their filters caused them serious harm. IVC filters are designed to reduce the risk of blood clots in people who are unable to take blood thinners, but lawsuits claim serious IVC filter complications.
Plaintiffs claim their IVC filter fractured and punctured veins and tissue after it migrated to other parts of the body. These medical devices damaged lungs, the heart and other organs. Lawsuits also detail how some filters, after migrating, were impossible to remove or caused organ perforation or organ damage.
C.R. Bard and Cook Medical were named as defendants in the largest share of legal claims about defective IVC filters. Both medical device companies settled thousands of claims, although more lawsuits are still winding their way through litigation after years of legal back-and-forth.
People also filed other IVC filter manufacturers, including ALN, Argon, Boston Scientific and CORDIS. None of these lawsuits were part of any multidistrict litigation as of February 2026.
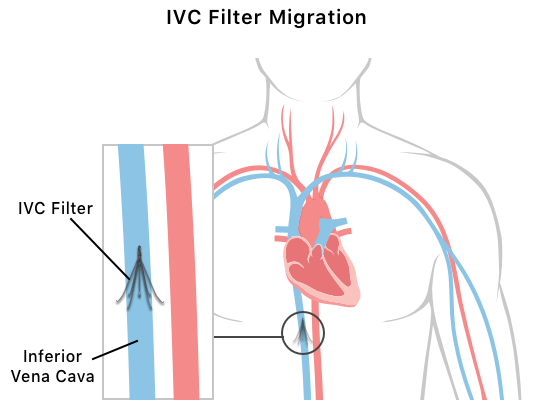
IVC Migration
Migration is a serious complication that occurs when an IVC filter has moved from its original implantation location. IVC filters get their name because of where they’re normally implanted — inside the inferior vena cava vein, which carries blood to the heart. But some IVC filters may move from the inferior vena cava to multiple other places in the body, including pulmonary arteries, hepatic veins, other veins and the heart.
Migration rates of various IVC filters range from 0% to 11.8%. What makes migration problematic is that filters can be hard to retrieve once they move, especially if they break inside the body. Fragmenting makes retrievals difficult. Success rates for finding and removing IVC filter fragments range from 50% to 100%.
Retrieval surgery is complex and can cause IVC filters to fracture, or break. Sometimes, retrieval is impossible. Both retrieval and inability to retrieve can pose serious risks.
Doctors have documented successful fragment removals from the IVC filter site and also from the pulmonary artery, aorta and right ventricle. Complications from migration surgery include stroke, pulmonary infarction and bacteremia.
Manufacturers and Brands Named in Lawsuits
Bard, Cook and Cordis are manufacturers that have multiple models of IVC filters named in legal claims. Cordis and Cook have two models named. Bard has six, according to available filings.
Plaintiffs allege at least one IVC filter model from three other device makers — ALN, Argon and Boston Scientific — was defective. However, neither Cordis nor ALN, Argon and Boston Scientific are part of an IVC filter MDL as of February 2026.
| MANUFACTURER | MODELS NAMED IN LAWSUITS |
|---|---|
| ALN | Optional |
| Argon | Option Elite |
| Bard | Recovery, G2, G2 Express (G2X), Eclipse, Denali, Meridian |
| Boston Scientific | Greenfield |
| Cook Medical | Celect, Gunther Tulip |
| Cordis | TrapEase, OptEase |
Federal lawsuits filed against Bard and Cook and other IVC filter manufacturers allege that defects in the design of IVC filters make them more likely to fracture, migrate, tilt or perforate the blood vessel. People who were injured say the companies knew or should have known about the dangers but failed to warn doctors and patients of the risks.
During a 2015 investigation of Bard IVC filters, NBC News reported that Bard executives had been aware of the risks for years, but the company did nothing. NBC obtained a 2004 Bard-commissioned study that found the Bard Recovery IVC filter had a higher failure rate than competing devices. NBC linked Bard IVC filters to 39 deaths.
Bard’s IVC Filter Lawsuits
Jury verdicts in Bard IVC lawsuits have found in favor of plaintiffs, awarding compensatory damages and ruling that Bard was negligent. A federal jury in Portland, Oregon, for example, ruled that Bard’s filter had a negligent design, and that the company did not warn doctors and patients about migration and perforation risks.
- $926,000: In May 2021, an Oregon jury ruled in favor of plaintiff Justin Peterson’s claim of damage to his internal vena cava and duodenum after a Bard Eclipse Filter failed.
- $3.6 million: In April 2018, the Ninth Circuit ruled in favor of the plaintiff in the first Bard IVC Filter bellwether trial, awarding $1.6 million in actual damages and $2 million in punitive damages to Sherr-Una Booker.
Juries award punitive damages to punish a defendant — in this case, Bard — when their actions cause severe harm. Booker’s lawyer argued Bard executives knew their product was dangerous but continued selling it without warning doctors about its risks.
Boston Scientific Greenfield IVC Filter Lawsuits
Boston Scientific is defending multiple individual lawsuits about its Greenfield IVC filter. It won one case in a summary judgment in October 2022 against plaintiff John Fuss.
The company is still fighting a claim from Kentucky resident Katherine Milan related to her Greenfield filter implant, which became occluded. She sued in 2016.
Cook IVC Filter Lawsuits
As of February 2026, there were nearly 8,000 active IVC lawsuits against Cook Medical in a federal MDL in the Southern District of Indiana. The company has found mixed results in court.
In December 2022, the Seventh Circuit ruled in favor of two Cook IVC filter plaintiffs, reversing the MDL judge who had dismissed their claim citing the statute of limitations. After Cook won its first bellwether trial in November 2017, a judge threw out the second bellwether based on an Indiana statute of limitations. However, an appellate court reversed that decision because courts previously allowed similar plaintiffs in an MDL.
In May 2018, a Texas jury awarded a $1.2 million verdict to Houston firefighter Jeff Pavlock, who claimed a Cook Celect IVC filter damaged his aorta and small intestine. The lawsuit was not part of the bellwethers. Cook is appealing.
IVC Filter Recalls and FDA Actions
Device makers issued seven IVC filter recalls between 2005 and 2019. Two actions were serious Class I recalls — the 2013 Cordis recall of 33,000 of its OptEast IVC filters and the 2005 Boston Scientific recall of 18,000 Greenfield filters.
The OptEase recall, to correct labeling and prevent backwards insertion of the devices, is the largest to date. The last IVC filter recall came in 2019 from Cook, which brought back multiple sets of its Gunter Tulip IVC because of labeling issues.
Questions IVC Filter Attorneys May Ask
What kind of problems has your IVC filter had?
Thousands of IVC filters have been recalled because they were defective, failed to open, tilted or migrated. Be sure to let your attorney know if your device malfunctioned.
When was your IVC filter implanted?
Most people filing lawsuits had their IVC filters implanted before 2003. Your IVC filter lawyer will want to review your medical records to confirm the date of your procedure.
What company made your IVC filter?
C.R. Bard and Cook Medical are responsible for making the majority of IVC filters being named in lawsuits. However, people have filed individual lawsuits against other IVC filter makers, including Boston Scientific and Cordis. The surgeon who performed your procedure should be able to tell you who made your device.
How have your IVC filter complications impacted your daily life?
Complications associated with IVC filters can have a physical and emotional impact on your life. Your attorney will want to know the degree to which your IVC filter complications have affected your ability to do your daily tasks.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.