Tylenol
The popular pain reliever Tylenol is found in more than 600 prescription and over-the-counter medications. While Tylenol is relatively safe when taken at the correct dosage, ingesting too much of the drug can lead to serious complications, including liver failure.
- Medically reviewed by Michael Gabay, Pharm.D., JD, BCPS, FCCP
- Last update: March 12, 2025
What Is Tylenol?
Tylenol (acetaminophen) is the most popular over-the-counter (OTC) pain relief medication used in the U.S. and around the world. Acetaminophen is the active ingredient in Tylenol and the generic name of the drug that is commonly found in other medications.
The drug was first sold in 1955 as Tylenol Elixir for Children, and today millions of American adults and children use the drug every week for common ailments such as head and body aches, colds, and fevers. In fact, according to the Consumer Healthcare Products Association, about 52 million consumers in the U.S. use products containing Tylenol each week.
Manufactured by Johnson & Johnson subsidiary McNeil Laboratories, the drug generates more than $1 billion a year. It is marketed as an effective painkiller that is safer than non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin or ibuprofen, which are associated with stomach discomfort or bleeding.
Tylenol Uses
Tylenol belongs to a class of drugs called analgesics and antipyretic agents. An analgesic relieves pain. An antipyretic reduces fevers. For more than 50 years, doctors have recommended Tylenol for both uses.
Unlike other analgesics like aspirin and ibuprofen (Advil, Motrin), Tylenol does not treat inflammation. It is most effective for minor aches and pains but can be used safely for long-term chronic pain such as arthritis. In fact, the American College of Rheumatology recommends Tylenol to treat arthritis, and it is especially useful in types of arthritis that are not accompanied by inflammation, like osteoarthritis.
Common Side Effects of Tylenol
Tylenol on its own is a non-prescription or over-the-counter medication. While prescription medications are required to include certain prescribing information, such as common side effects, non-prescription medications like Tylenol do not typically contain the same information. Therefore, much of the information regarding side effects provided for the over-the-counter medication refers to serious side effects of Tylenol, usually because of overdose.
Common Side Effects of Acetaminophen Include:
- Constipation (infrequent or difficult to pass bowel movements)
- Agitation
- Headaches
- Insomnia (trouble falling asleep or staying asleep)
- Vomiting
Serious side effects of acetaminophen are rare when taken as directed. It’s typically well-tolerated in both adults and children.
Findings from one 2022 clinical research trial suggested that regular daily intake of 4 g acetaminophen increased systolic blood pressure in individuals with hypertension by about 5 mm Hg compared with a placebo. The study concluded that this increase in cardiovascular risk calls into question the safety of regular acetaminophen use in similar situations.
Complications Associated with Tylenol
Tylenol is associated with serious complications in the cases of overdose and long-term use, including liver damage and rare but dangerous skin reactions. It is the leading cause of acute liver failure in the U.S., and the drug in some cases has led to fatalities. The active ingredient in Tylenol, acetaminophen, accounts for more than 100,000 calls to poison centers, roughly 60,000 emergency-room visits and hundreds of deaths each year in the U.S. In England, it is the leading cause of liver failure requiring transplants.
“Acetaminophen is a dangerous drug,” Dr. John Brems, professor of surgery and chief of intra-abdominal transplantation at Loyola University in Chicago told ABC News. “Many of these patients took acetaminophen in addition to alcohol. I end up transplanting three to four patients per year, and two to three die before we can transplant them. It is probably the most dangerous OTC drug in this country.”
However, while a study from the University of Edinburgh published in February 2022 did find risk of increased blood pressure from long-term use, Dr Iain MacIntyre, the lead investigator and a consultant in clinical pharmacology and nephrology at NHS Lothian, noted, “This is not about short-term use of paracetamol for headaches or fever, which is, of course, fine – but it does indicate a newly discovered risk for people who take it regularly over the longer term, usually for chronic pain.”
Taking Tylenol during pregnancy may increase the risk of children developing autism or attention-deficit/hyperactivity disorder, or ADHD. Current research is limited, and the results are mixed.

-
2009
FDA issued guidelines for adding overdose information to products.
-
2011
The agency confirmed the link between acetaminophen and liver damage and added a black box warning, the agency’s strongest warning, to the label of prescription products containing the drug.
-
2011
FDA asked drug manufacturers to limit the strength of acetaminophen in prescription drug products to 325 mg.
-
2011
The agency added a warning highlighting the potential for allergic reactions (e.g., swelling of the face, mouth, and throat, difficulty breathing, itching, or rash) to the label of all prescription drug products that contain acetaminophen.
-
2013
FDA released a safety warning about acetaminophen and rare but serious skin reactions.
-
2014
The agency announced its intent to take steps to withdraw approval of prescription combination drug products containing more than 325 mg of acetaminophen.
-
2015
FDA announces findings from review of acetaminophen use during pregnancy.
Tylenol Use During Pregnancy
The FDA reviewed possible risks of acetaminophen use during pregnancy and released its findings in a January 2015 Drug Safety Communication.
According to the safety communication, two U.S. studies indicate that 65 to 70% of pregnant women in the U.S. reported using acetaminophen anytime during pregnancy.
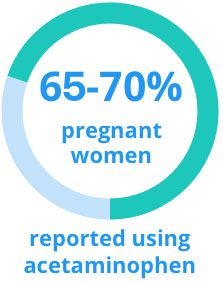
One study reported an increased risk between acetaminophen use in pregnancy and ADHD in children. Women reporting any acetaminophen use in pregnancy were also significantly more likely to have a child with a hyperkinetic disorder diagnosis or a child who used ADHD medications, compared to unexposed women.
Associations for all outcomes were strongest for acetaminophen use in multiple trimesters and for more than 20 weeks during pregnancy, the FDA noted.
The FDA said the study had a number of methodologic limitations and concluded, the weight of evidence is inconclusive regarding a possible connection between acetaminophen use in pregnancy and ADHD in children.
Since then, other studies have reported mixed results. A 2022 meta-analysis found an association between Tylenol and adverse neurodevelopmental outcomes.
Acetaminophen is still the safest known drug to take during pregnancy for problems like fever and pain, said Dr. Salena Zanotti, an OB/GYN at Cleveland Clinic. When you’re pregnant it’s riskier to have an untreated fever than it is to take acetaminophen.
People have filed Tylenol lawsuits claiming the drug caused children to develop autism and ADHD. A class-action lawsuit is moving through the court system under Judge Denise Conte.
Tylenol Recalls
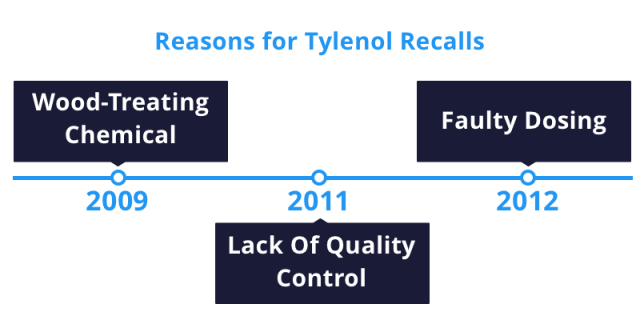
Tylenol faced a string of recalls from 2009 to 2012. Some Tylenol products didn’t return to store shelves until 2013.
In 2009, McNeil recalled many Tylenol brands because a chemical for treating wood made it into the medicine, causing nausea, vomiting and diarrhea. In 2011, it expanded the recall to include several more lots of the medication. The recall involved tens of thousands of Tylenol products and prompted Johnson & Johnson to close a manufacturing plant. Furthermore, the FDA stepped in to supervise quality control measures at three plants. In 2012, McNeil recalled nearly 600,000 bottles of infant Tylenol for faulty dosing systems that could result in babies receiving too little or too much medicine.
Chicago Tylenol Murders
The drug was also linked to several murders in 1982 called the Chicago Tylenol Murders. Several people in Chicago died after taking Extra Strength Tylenol caplets that were laced with cyanide. McNeil was not implicated in the murders because the bottles came from different factories, and all seven deaths took place in the Chicago area, ruling out the possibility of tampering during production.
After the murders, Johnson & Johnson sent warnings to hospitals and distributors, stopped all advertising and Tylenol production and recalled approximately 31 million bottles. The murders remain unsolved.
Other Examples of Tylenol Recalls
| Product | Reason | Date |
|---|---|---|
| TYLENOL®, Extra Strength Caplets, 225 count | Uncharacteristic musty, moldy odor | 6/28/11 |
| TYLENOL® 8 Hour, TYLENOL® Arthritis Pain, and TYLENOL® upper respiratory products, and certain lots of BENADRYL®, SUDAFED PE®, and SINUTAB® products | Insufficient development during manufacturing | 1/14/11 |
| Tylenol Cold Multi Symptom Daytime Liquid 8 oz Citrus Burst | Mislabeled - alcohol content not listed on front panel | 11/27/10 |
| Tylenol 8 Hour Caplets in 50 count bottles | Off odor - musty/moldy | 10/18/2010 |
Tylenol Forms and Doses
Tylenol is available in tablet, chewable tablet, capsule, suspension or solution (liquid), extended-release (long-acting) tablet and orally disintegrating tablet forms and as a suppository for rectal use. There is also an intravenous form of acetaminophen that is used in hospital settings.
Most formulations are available in several dose amounts, usually from 300 mg to 1000 mg. It comes in prescription and nonprescription strengths. However, the strength of acetaminophen in prescription drug products is limited to 325 mg per request by the FDA.
The maximum indicated dose in a 24-hour period should not exceed 4,000 mg for most patients. Taking more than 4,000 mg of Tylenol a day puts users at risk of liver failure. Experts say anyone taking Tylenol long-term for chronic pain should consider having liver function tests once or twice a year.
- Child-Specific Product
- To administer the medicine to children, use the child-specific product with a dosing cup or dropper that comes with it. Teaspoons found in the home vary in size and their use to administer Tylenol could overdose or underdose a child. You may need to shake the liquid before each use.
- Chewable Tablet
- The Tylenol Meltaways chewable tablet softens in the mouth to make it easier to chew. Chew the tablet thoroughly before swallowing it.
- Meltaways
- Meltaways are orally disintegrating tablets. Place the tablet in your mouth and either wait for it to dissolve or chew it before swallowing.
- Extended–Release Tablets
- Extended-release tablets are meant to be swallowed whole. Do not split, chew, crush or dissolve these tablets before swallowing.
Calling this number connects you with a Drugwatch.com representative. We will direct you to one of our trusted legal partners for a free case review.
Drugwatch.com's trusted legal partners support the organization's mission to keep people safe from dangerous drugs and medical devices. For more information, visit our partners page.


